Abstract
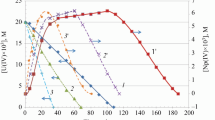
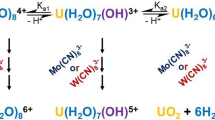
The kinetics of U(IV) oxidation with atmospheric oxygen in NaHCO3 solutions was studied by spectrophotometry. In 1 M NaHCO3 at [U(IV)]0 = 20 mM, an induction period is observed, which virtually disappears with decreasing [U(IV)]0 to 1.0 mM. The induction period is caused by the fact that initially U(IV) exists in a weakly active polymeric form. Addition of U(VI) to the initial solution accelerates the oxidation. In a 1 M NaHCO3 solution containing 0.1–1.0 mM U(IV), the U(IV) loss follows the first-order rate law with respect to U(IV) and O2. The pseudo-first-order rate constants, bimolecular rate constants, and activation energy of the U(IV) oxidation were calculated. In dilute NaHCO3 solutions (0.5–0.01 M), the hydrolysis and polymerization of U(IV) become more pronounced. The autocatalysis mechanism presumably involves formation of a complex [U(IV) · U(VI)] with which O2 reacts faster than with U(IV). Oxidation of U(IV) occurs by the two-electron charge-transfer mechanism.
Similar content being viewed by others
References
Kanevskii, E.A., Goncharov, V.V., and Rengevich, V.B., Radiokhimiya, 1965, vol. 7, no. 5, pp. 579–585.
Shilov, V.P., Yusov, A.B., Fedoseev, A.M., et al., Radiokhimiya, 2007, vol. 49, no. 5, pp. 412–416.
Tourné C. and Tourné, G., Bull. Soc. Chim. Fr., 1969, no. 4, pp. 1124–1136.
Bion, L., Moisy, Ph., and Madic, C., Radiochim. Acta, 1995, vol. 69, no. 4, pp. 251–257.
Nelson, F. and Kraus, K.A., J. Am. Chem. Soc., 1951, vol. 73, no. 5, pp. 2157–2161.
Wester, D.W. and Sullivan, J.C., Inorg. Chem., 1980, vol. 19, no. 9, pp. 2838–2840.
Fedoseev, A.M., Peretrukhin, V.F., and Krot, N.N., Dokl. Akad. Nauk SSSR, 1979, vol. 244, no. 5, pp. 1187–1190.
Berger, P., Blank, P., and Bourges, J., Radiochim. Acta, 1988, vol. 43, no. 4, pp. 217–228.
Pikaev, A.K., Shilov, V.P., and Gogolev, A.V., Usp. Khim., 1997, vol. 66, no. 9, pp. 845–879.
Bakac, A. and Espenson, J.H., Inorg. Chem., 1995, vol. 34, no. 7, pp. 1730–1735.
Groisman, A.Sh. and Khomutov, N.E., Usp. Khim., 1990, vol. 59, no. 8, pp. 1217–1250.
Rai, D., Felmy, A.R., Hess, N.J., et al., Radiochim. Act, 1999, vol. 82, no. 1, pp. 17–25.
Ho, C.H. and Miller, N.H., J. Colloid Interface Sci., 1986, vol. 113, no. 1, pp. 232–240.
Solubility Data Ser., vol. 7: Oxygen and Ozone, Battino, R., Ed., Oxford: Pergamon, 1981.
Bratsch, S.G., J. Chem. Phys. Ref. Data, 1989, vol. 18, no. 1, pp. 1321.
Author information
Authors and Affiliations
Additional information
Original Russian Text © V.P. Shilov, A.B. Yusov, A.M. Fedoseev, V.F. Peretrukhin, C.H. Delegard, 2008, published in Radiokhimiya, 2008, Vol. 50, No. 5, pp. 397–402.
Rights and permissions
About this article
Cite this article
Shilov, V.P., Yusov, A.B., Fedoseev, A.M. et al. Oxidation of uranium(IV) with oxygen in NaHCO3 solution. Radiochemistry 50, 460–465 (2008). https://doi.org/10.1134/S1066362208050044
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362208050044