Abstract
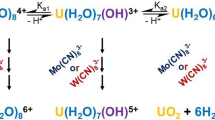
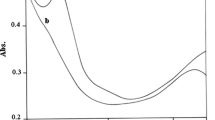
Kinetic features of the chemiluminescence accompanying oxidation of U(IV) with xenon difluoride in solutions of 0.2 M H2SO4 in H2O and 0.2 M of D2SO4 in D2O at temperatures within 283–313 K and concentrations (M) 10−6 ≤ [U(IV)] ≤ 10−4 and 10−4 ≤ [XeF2] ≤ 10−3 were examined. The shape of the kinetic curve depends on the concentration of the reactants and the solution temperature. At [U(IV)] = 10−6 and [XeF2] = 10−4 M, the curve exhibits a maximum, more prominent at low temperatures. At relatively high concentration ([U(IV)] = 10−4 and [XeF2] = 10−3 M), self-acceleration of the reaction is observed: The mechanism of oxidation of U(IV) turns into a branched-chain mode owing to a higher concentration in the solution of radicals ·OH, HO ·2 , and XeF ·2 , whose additional amounts are yielded by hydrolytic reduction of XeF2. A kinetic isotope effect of the solvent k H/k D was revealed, which attains a maximum of 1.8 at 283 K. The activation energies of the oxidation of U(IV) with xenon difluoride in H2SO4 and D2SO4 solutions were estimated at E a,H = 12 and E a,D = 13 kcal mol−1, respectively. The occurrence of the isotope effect is an indirect evidence of participation in the reaction of the OH (or OD) groups. The rate of hydrolytic reduction of XeF2 in deuterated solvent (0.2 M D2SO4 in D2O) in its photochemical stage is several times lower, and the luminescence accompanying the reaction is by an order of magnitude smaller than that in 0.2 M H2SO4.
Similar content being viewed by others
References
Kazakov, V.P., Parshin, G.S., Gusev, Yu.K., et al., Dokl. Akad. Nauk SSSR, 1978, vol. 239, no. 6, p. 1397.
Kazakov, V.P., Khemilyuminestsentsiya uranila, lantanoidov i d-elementov (Chemiluminescence of Uranyl, Lanthanides, and d Elements), Moscow: Nauka, 1980.
Gusev, Yu.K., Kazakov, V.P., Khamidullina, L.A., et al., Khimiya urana (Chemistry of Uranium), Laskorin, B.N., Ed., Moscow: Nauka, 1981, p. 264.
Khamidullina, L.A., Lotnik, S.V., and Kazakov, V.P., Radiokhimiya, 1998, vol. 40, no. 3, p. 203.
Lotnik, S.V., Khamidullina, L.A., and Kazakov, V.P., Dokl. Ross. Akad. Nauk, 1999, vol. 366, no. 3, p. 345.
Lotnik, S.V., Khamidullina, L.A., and Kazakov, V.P., Dokl. Ross. Akad. Nauk, 1999, vol. 366, no. 4, p. 493.
Khamidullina, L.A., Lotnik, S.V., and Kazakov, V.P., Radiokhimiya, 2004, vol. 46, no. 2, p. 123.
Lotnik, S.V., Khamidullina, L.A., and Kazakov, V.P., Radiokhimiya, 2006, vol. 48, no. 3, p. 229.
Appelman, E.H. and Malm, J.G., J. Am. Chem. Soc., 1964, vol. 86, no. 11, p. 2297.
Kazakov, V.P., Tolstikov, G.A., Lotnik, S.V., et al., Izv. Akad. Nauk SSSR, Ser. Khim., 1984, no. 12, p. 2829.
Lotnik, S.V., Khamidullina, L.A., and Kazakov, V.P., Radiokhimiya, 1993, vol. 35, no. 2, p. 50.
Melander, L. and Saunders, W.H., Jr., Reaction Rates of Isotopic Molecules, New Yprk: Wiley, 1980.
Treindl, L. and Adamčikova, L., Chem. Zvesti, 1973, vol. 27, no. 4, p. 433.
Burrows, H.D. and Kemp, T.J., Chem. Soc. Rev., 1974, vol. 3, no. 2, p. 139.
Khamidullina, L.A., Lotnik, S.V., and Kazakov, V.P., Radiokhimiya, 1998, vol. 40, no. 3, p. 208.
Appelman, E.H., Inorg. Chem., 1967, vol. 6, no. 7, p. 1305.
Goncharov, A.A., Kozlov, Yu.N., and Purmal’, A.P., Zh. Fiz. Khim., 1981, vol. 55, no. 7, p. 1623.
Koltunov, V.S., Kinetika reaktsii aktinoidov (Kinetics of Reactions of Actinides), Moscow: Atomizdat, 1974, p. 312.
Lotnik, S.V., Khamidullina, L.A., and Kazakov, V.P., Izv. Ross. Akad. Nauk, Ser. Khim., 2000, no. 9, p. 1522.
Klimina, S.N., Mamykin, A.V., and Kazakov, V.P., Dokl. Ross. Akad. Nauk, 2003, vol. 392, no. 5, p. 638.
Bakac, A. and Espenson, J.H., Inorg. Chem., 1995, vol. 34, no. 7, p. 1730.
Pikaev, A.K. and Kabakchi, S.A., Reaktsionnaya sposobnost’ pervichnykh produktov radioliza vody (Reactivity of the Primary Products of Water Radiolysis), Moscow: Energoizdat, 1982.
Newton, T.W. and Baker, F.B., Inorg. Chem., 1965, vol. 4, no. 8, p. 1166.
Frolov, A.A. and Rykov, A.G., Radiokhimiya, 1979, vol. 21, no. 3, p. 329.
Author information
Authors and Affiliations
Additional information
Original Russian Text © S.V. Lotnik, L.A. Khamidullina, V.P. Kazakov, 2007, published in Radiokhimiya, 2007, Vol. 49, No. 6, pp. 513–519.
Rights and permissions
About this article
Cite this article
Lotnik, S.V., Khamidullina, L.A. & Kazakov, V.P. Formation of excited uranyl ion in oxidation of U(IV) with xenon difluoride in aqueous solutions of sulfuric and deuterated sulfuric acids. Radiochemistry 49, 586–592 (2007). https://doi.org/10.1134/S1066362207060082
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1066362207060082