Abstract
Pseudostellaria heterophylla of the Caryophyllaceae family is an important medicinal herb in traditional Chinese medicine, but it encounters continuous cropping obstacle during cultivation. This study aimed to study the differences in the response of Pseudostellaria heterophylla cultivation to soil microorganisms and phenolic substances in different types of soil (Loamy soil and Clayey soil). The analysis of soil metabolites using HPLC-ESI-Q/TOF-MS and HPLC-DAD techniques revealed that the content of various phenolic components in the rhizosphere soil increased significantly after Pseudostellaria heterophylla cultivation. Pseudostellaria heterophylla cultivation in yellow soil could increase the abundance of Proteobacteria, Ascomycota, and Thermoplasmatota, while in brown soil, the abundance of Ascomycota significantly decreased. In addition, Pseudostellaria heterophylla cultivation could increase the α-diversity of bacteria and fungi in yellow soil, while reducing the α-diversity of archaea, and the impact on diversity in brown soil was relatively small. Correlation analysis showed that phenolic compounds were more likely to regulate the genera Bradyrhizobium, Calcarisporiella, Boothiomyces and Methanocella. Pseudostellaria heterophylla may regulate rhizosphere microbial diversity and community structure by secreting phenolic. However, in different types of soil environments, the response mechanism of soil microorganisms to Pseudostellaria heterophylla planting might vary. Our study provides new insights that the variability in the response of different soil types to Pseudostelariae heterophylla cultivation should be taken into account when developing strategies for reducing continuous cropping obstacles in medicinal plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Pseudostellaria heterophylla (Miq.) Pax ex Pax et Hoffm, a plant of the Caryophyllaceae family, has a long medicinal history in China. It was cultivated in regions such as Zherong of Fujian, Jurong of Jiangsu, Shibing of Guizhou, and Xuancheng of Anhui. Due to its rich medicinal and nutritional value, it has been added to the “List of Chinese Medicinal Materials for Health Food Use” [3, 7, 9, 12]. However, as the planting area of Pseudostellaria heterophylla continues to expand, issues with continuous cropping obstacles have become more prominent, leading to a degradation in quality and a decrease in yield, which severely impacts the development of the Pseudostellaria heterophylla industry [30, 35]. Research has shown that the occurrence of continuous cropping obstacles in Pseudostellaria heterophylla was linked to the secretion of root exudates and the resulting variation in rhizosphere microorganisms [21, 34, 36].
Phenolic acids were known to be the most important regulators of the soil microbiome [2, 22]. Changes in the phenolic acid profile in soil drove the succession of microbiomes, and modifying phenolic acids could alter the structure of soil bacterial communities [26, 39]. Moreover, the interplay between soil types and microorganisms is complex. Studies indicate that specific microorganisms may exhibit preferences for particular soil types, where the physicochemical properties of the soil, such as texture and pH, play a pivotal role in shaping microbial communities [11, 15]. As the response of microorganisms' functional groups to environmental factors varies, we speculate that different soil types will uniquely impact the microbial community composition in inter-rhizosphere soils of Pseudostellaria heterophylla ginseng. Consequently, further research is needed to explore how cultivating Pseudostellaria heterophylla in various soil types affects the structure and diversity of soil bacteria, fungi, and archaea community. Such research is essential to comprehensively assess the influence of Pseudostellaria heterophylla cultivation on soil microorganisms and autotoxins across diverse soil types.
To our knowledge, there is a lack of research that simultaneously compares the composition and correlation with autotoxin substance content of bacterial, fungi and archaeal community in different soil types. In this study, HPLC-ESI-Q/TOF-MS and HPLC-DAD technology were used to identify and analyze phenolic autotoxic substances in the rhizosphere soil of Pseudostellaria heterophylla, while Illumina MiSeq sequencing was used to elucidate the abundance, structure and composition of bacterial, fungi and archaeal communities. We hypothesize that (1) Planting Pseudostellaria heterophylla will affect the biodiversity and community structure of various microorganisms (including bacteria, fungi, and archaea) in the rhizosphere soil to varying degrees by secreting autotoxin substances; (2) Under different soil conditions, the mechanism of disturbance of the soil microbial ecosystem caused by Pseudostellaria heterophylla planting may vary.
MATERIALS AND METHODS
Field experiments and soil sampling. Two typical soils with different physical and chemical properties were collected in Zherong County, Fujian Province, China: Loamy soil (Yellow in colour, called yellow soil; 119°49′27″ E, 27°17′22″ N; 119°49′16″ E, 27°17′15″ N; 119°48′44″ E, 27°17′11″ N), and Clayey soil (Brown in color, called brown soil; 119°49′8″ E, 27°15′43″ N; 119°48′38″ E, 27°14′30″ N; 119°49′30″ E, 27°16′8″ N). These locations are all newly reclaimed land, with the first year of planting Pseudostellaria heterophylla, and adjacent fallow plots as a control. The annual precipitation in the region is 1700–2300 mm, with a frost-free period of 238 days and an average annual temperature of 13–18°C. Seedlings of Pseudostellaria heterophylla were sown on 16–18 November 2020 and soil samples were collected on 15 July 2021. A forked spade was used to uproot the plant from the soil, and the rhizospheric soil was then collected by gently brushing off the soil that was densely attached to the rhizomes and roots. Five soil samples were collected from each plot following a W-scheme sampling method. At each tip of the W-shaped path, one soil sample was taken, and subsequently, all five samples from each plot were pooled and combined for further analysis. After collecting the rhizosphere soil of Pseudostellaria heterophylla, the first part was used to determine the soil moisture content. The second part was quickly refrigerated at –80°C to extract microbial DNA, and the third portion was used to analyze the physicochemical properties of the soil and determine the levels of autotoxin substances.
Soil physicochemical characteristics. The soil moisture content (SMC) was determined through a gravimetric approach by subjecting fresh soil samples to constant weight at 105°C. The measurement of soil pH was conducted in a 1 : 2.5 (soil: water) suspension utilizing a glass electrode [6]. The analysis of available nitrogen, available phosphorus, and available potassium in the soil followed the methodologies outlined in a prior study [29, 39].
Fingerprinting of soils. Fingerprinting technology can reflect the types and quantities of chemical components in samples [36]. The dried soil sample weighing 20 g was weighed and transferred into a 100 mL conical flask, followed by the addition of 50 mL of 70% methanol to the flask. The mixture was thoroughly shaken to ensure proper mixing. Following this, the flask was subjected to ultrasonic extraction (500 W, 40 KHz) for a duration of 1 h. It was allowed to stand overnight, after which centrifugation (3500× g, 15 min) was performed; the same steps were repeated with the resulting residue. The solutions acquired from the two centrifugation processes were merged and dried using a rotary evaporator (BÜCHI Labortechnik, R-300, Switzerland). Subsequently, the resulting residue was dissolved in 2 mL of ultrapure water. A Dionex Ultimate 3000 HPLC system (Thermo Fisher Scientific, Germering, Germany) was used for HPLC analysis, equipped with a SinoChrom ODS-BP RP column (5 µm, 250 × 4.6 mm); the column temperature was set at 30°C; the injection volume was set at 5 µ L; the detection wavelength was set at 230 nm. The mobile phase comprised a mixture of water with 0.1% acetic acid and methanol, the flow rate was set at 1 mL/min. The program for the gradient elution was carried out as follows: 10.0 min, 15% methanol; 60.0 min, 70% methanol.
HPLC-ESI-Q/TOF-MS analysis. The experiment was performed on an HPLC-ESI-Q/TOF-MS system (Agilent, Model G6520B, USA). In the chromatographic separation part, parameters such as the type of chromatographic column, column temperature, mobile phase, and elution program should remain consistent with the parameters under “fingerprinting of soils”. The mass spectrometry analysis utilized electrospray ionization (ESI) in negative ion mode, with the following parameters: capillary voltage at 3500 V, fragmentor at 100 V, skimmer at 65 V, octopoleRFPeak at 750 V, nebulizer pressure at 40 psi, drying gas temperature at 350°C, and gas flow at 10 L/min. Data were collected within a mass range of m/z 50–1000, and collision energies were set at 20 V. The TOF analyzer’s precise mass capability ensured reliable identification of detected metabolites, with routine analysis yielding mass errors below 5 ppm. The MassHunter Workstation software was utilized to manage acquisition parameters and process the acquired data from the HPLC-Q-TOF-MS system.
Quantitative analysis of soil phenolic acids. 25 g of dried soil was taken and placed in a 500 mL conical flask. It was then treated with 200 mL of 1 mol/L NaOH solution, well-shaken, and subjected to ultrasonic extraction for 50 min. The filtrate was subjected to centrifugation to isolate its components, with the supernatant being collected. The pH of the supernatant was lowered to 2.5 through the addition of hydrochloric acid, and allowed to stand overnight for further processing. A second centrifugation step was then performed, with the supernatant transferred to a 500 mL separatory funnel. Ethyl acetate solution (200 mL) was added for extraction, which was repeated twice to obtain a combined ethyl acetate layer. This layer was dried using a rotary evaporator and the remaining solids dissolved in 5 mL of methanol. The solution was filtered through a 0.45 μm pore size filter membrane for HPLC analysis. Standard solutions with an initial concentration of 1 mg/mL were prepared by accurately weighing ten standards (Gallic acid, Protocatechuic acid, 4-Hydroxybenzoic acid, Vanillic acid, Syringic acid, Vanillin, p-Coumaric acid, Ferulic acid, Benzoic acid, Salicylic acid) and dissolving them in a methanol aqueous solution. Following this, the solutions were diluted into six different levels, creating consecutive working standard solutions. The chromatographic conditions are as follows Chromatographic column: Hypersil ODS2 RP-column (5 μm, 150 × 4.0 mm); isocratic elution (methanol : 0.1% acetic acid = 17 : 83); wavelength of detection: 280 nm; column temperature: 35°C; flow rate: 0.6 mL/min; injection volume: 10 μL. To assess the precision of the method, both intra-day and inter-day analyses were performed. For intra-day precision, the same sample solution was analyzed six times within a single day, while for inter-day precision, the sample solution was analyzed on three successive days. The repeatability was assessed by the analysis of six sample solutions prepared independently of each other. The sample solution was analyzed at different time points (0, 2, 4, 8, 16 and 24 h) after storage at room temperature to determine the constancy of the sample solution. For the generation of standard curves, a series of concentration standard solutions were analyzed by the HPLC system, with peak areas plotted against the concentrations.
DNA extraction and amplicon sequencing. We utilized Illumina-based sequencing to assess the diversity of soil archaea, bacteria, and fungi [5]. For amplification, the archaeal 16S rRNA gene V3 to V5 regions were targeted using the pair Arch915R and Arch344F, whereas the bacterial 16S rRNA gene V3 to V4 regions were targeted using the pair 806R and 338F. Additionally, the primer pair ITS1F and ITS2 was used to amplify the fungal internal transcribed spacer (ITS) region 1. Extraction and paired-end sequencing of microbial genomic DNA was performed on an Illumina MiSeq platform at the Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China), following standard protocols.
Analyzing of sequencing data. Soil physicochemical properties and microbial diversity were analyzed using t-tests, and one-way ANOVA was used during phenolic acid-microbial association analysis. For all analyses, p < 0.05 was considered statistically significant. For the analysis of sequence data, the Major Bio Cloud Platform (https://cloud.majorbio.com/) was employed, which is based on silva and Unit databases. Principal co-ordinates analysis (PCoA) based on Bray-Curtis dissimilarity was conducted to assess differences in the rhizosphere soil. To test the significance, permutational multivariate ANOVA was applied. The co-occurrence networks between microorganisms and phenolic acids were analyzed using non-parametric Spearman correlation with a significance threshold of p < 0.01 and an absolute value of rs > 0.5. The co-occurrence networks were visualized using the Gephi interactive platform.
RESULTS AND DISCUSSION
Physicochemical Characteristics analysis of soil. Soil chemical properties are closely related to crop growth and development and are important indicators for measuring soil fertility [8, 24, 28]. Table 1 shows the chemical properties of the two different soil types from this study. Both yellow soil and brown soil produced varying degrees of change in soil nutrient content after Pseudostellaria heterophylla planting compared to control soils. From Table 1 we can see that the content of available nitrogen, available phosphorus and available potassium in the soil increased significantly after Pseudostellaria heterophylla cultivation in the two different soil types. Therefore, the soil fertility factor (AN/AK/AP) does not seem to be a key factor causing the continuous crop barrier in Pseudostellaria heterophylla. No significant change in soil moisture content was observed in both yellow and brown soils, while pH values decreased significantly, suggesting that soil acidification may be an influential factor driving barriers to crop succession after Pseudostellaria heterophylla cultivation.
Fingerprint analysis by HPLC. To explore the material basis for causing soil acidification after Pseudostellaria heterophylla cultivation, we developed HPLC fingerprints of yellow soil and brown soil. The chromatographic separation method, column temperature, detection wavelength and other parameters were optimized to obtain the richest material information and the best peak separation in the two soil types. After several attempts, the best gradient elution conditions were finally determined and a better chromatogram (Fig. 1) was obtained. For yellow soil, two new peaks at 15 and 40 min were evident in the soil planted with Pseudostellaria heterophylla compared to the control soil, and the peak areas of the two peaks with retention times at 35 and 38 min were significantly increased. No significant new peaks were observed for brown soil, but a significant increase in peak area was observed for several peaks. These results suggest that cultivation of Pseudostellaria heterophylla might cause changes in the composition and/or proportions of chemical constituents in their rhizosphere soil soils.
Identification of metabolites in the rhizosphere soils. In order to clarify the information on the chemical structure of the different peaks in the fingerprint profile of the soil after Pseudostellaria heterophylla cultivation, the soil samples were analyzed using HPLC-ESI-Q/TOF-MS techniques [14, 20, 27]. The soil sample was analyzed in negative ion mode, and through the examination of fragment information, retention time, and comparison with the database, a tentative identification of 34 chemical constituents was achieved. These specific chemical constituents are documented in Table 2. Interestingly, we found eight phenolic acid components in the soil after planting Pseudostellaria heterophylla, and their levels were significantly increased compared to the control soil. These results are an indication that the accumulation of phenolic acid components may be the material basis for causing soil acidification and thus inducing continuous crop failure.
Changes in the content of phenolic acid components in rhizosphere soil soils. Based on the mass spectrometry results and in conjunction with literature reports, we selected ten phenolic acids components for quantitative analysis [10, 16, 25]. To quantify the changes in the phenolic acid content of the soil after planting Pseudostellaria heterophylla in different soil types, an HPLC-DAD method was designed for the quantification of phenolic acid content in the rhizosphere soil. To validate the HPLC method, precision, stability, repeatability, and recovery were examined. In Fig. 2, the HPLC-DAD chromatograms of both a representative sample and the mixed standard solution are presented, demonstrating the effective separation of the ten phenolic acids under the applied experimental conditions. Based on the analysis of the chromatograms, it was observed that the separation of compounds with adjacent peaks was greater than 1.83. Additionally, the plate number of the column has been computed, considering the ten compounds presented in Fig. 2b, demonstrated a variation spanning from 5244 to 102 715, with the trailing factor falling within the range of 0.96 to 1.03.
Alterations in the concentrations of 10 phenolic acids in soil post-planting of Pseudostellaria heterophylla in various soil types. (a) 1–10 in order of Gallic acid, Protocatechuic acid, 4-Hydroxybenzoic acid, Vanillic acid, Syringic acid, Vanillin, p-Coumaric acid, Ferulic acid, Benzoic acid, Salicylic acid. the y-axis represents the difference in phenolic acid content in the soil before and after the introduction of Pseudostellaria heterophylla. (b) Typical chromatograms of HPLC for determination of phenolic acid content in different soil samples. SC presents the chromatograms of 10 phenolic acid standards.
To assess the precision of the equipment, the standard solution underwent six consecutive analyses on the same day and was further analyzed on three consecutive days. The intraday analyses revealed that the retention time (Rt) and peak area of the samples showed relative standard deviation (RSD) values within the range of 0.02 to 0.11% and 0.63 to 1.69%, respectively. The high precision of the instrument is demonstrated by these results. Stability assessment involved measuring the liquid chromatographic performance of the same sample at different points in time (0, 2, 4, 8, 16, and 24 h), demonstrates excellent stability within 24 h with Rt and peak area RSD values of less than 0.22 and 3.16% respectively. The RSD values for Rt and peak area were all less than 0.33 and 4.46% respectively when five independent solutions of the same sample were analyzed to assess repeatability. These results confirm the reliable repeatability of the instrument. The recoveries of the seven components from the soil sample ranged from 95.65 to 102.46%, indicating good accuracy in the quantitative determination.
The simultaneous determination of the ten phenolic acids in the rhizosphere soil was carried out using the established HPLC-DAD method. The results are summarized in Fig. 2a. Overall, there was an increasing trend in the phenolic acid content of both the yellow soil and brown soils after the planting of the Pseudostellaria heterophylla compared to the control soils. The planting of Pseudostellaria heterophylla increased the content of vanillin, p-coumaric acid and ferulic acid by 1.06, 14.91 and 0.81 mg/kg in the yellow soil and by 3.12, 3.86, and 0.29 mg/kg in the brown soil. The major increase in phenolic acid in the yellow soil was p-coumaric acid, whereas the major increase in the brown soil was vanillic acid, suggesting that the driving factors causing soil acidification in these two soils are different, with acidification in the yellow soil being mainly caused by p-coumaric acid, whereas in the brown soil it may be mainly caused by vanillic acid. At the same time, we observed significantly lower levels of 4-hydroxybenzoic acid and vanillic acid in yellow soil. These phenomena suggest that there may be significant differences in the response mechanisms that cause Pseudostellaria heterophylla to exhibit continuous crop obstacle in different soil types.
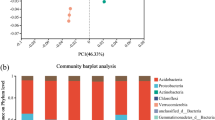
The effect of Pseudostellaria heterophylla cultivation on microbial diversity and function in different soil types. Soil microorganisms play a fundamental role within soil ecosystems in processes such as the decomposition and conversion of organic matter, the cycling and utilization of nutrients, and the establishment of soil fertility [17, 23, 39]. In order to explore the effects of Pseudostellaria heterophylla cultivation on the composition and structure of soil bacteria, fungi and archaea in different soil types, precise taxonomic classifications of microbial species and estimations of Alpha-diversity were conducted using OTU data obtained from the soil samples, employing a dissimilarity level of 3%. The raw fastq files were demultiplexed and quality filtered using QIIME (version 1.9.1). After filtering and clustering with 97% similarity, each sample obtained 908 ± 96 OTUs for bacteria, 174 ± 21 OTUs for fungi, and 100 ± 38 OTUs for archaea. The abundance of OTUs for bacteria and fungi was significantly higher in AY (Yellow soil after planting) practice compared to BY (Yellow soil before planting). Specifically, the number of bacterial OTUs in AY (2406) was 22% higher than the number of bacterial OTUs in BY (1971). We used principal co-ordinate analysis to determine the difference in beta diversity before and after Pseudostellaria heterophylla cultivation in different soil types (Fig. 3a). We found significant differences in the PC1 direction among the three microbial group. Venn diagrams were utilized to visually represent the intersection of operational taxonomic units (OTUs) among different microbial groups (Fig. 3b). In the case of archaea, the analysis identified 133 and 232 archaeal OTUs in AY and BY, respectively. Among these, only 103 OTUs were common to both AY and BY treated soils, while most of the remaining OTUs (129) were exclusive to BY treated soil. In contrast, no significant changes were found in the OTUs numbers of these three microbial group for AB (Brown soil after planting) compared to BB (Brown soil before planting).
Alpha diversity serves as a metric to assess the intricacy of species diversity within a given sample [4, 33]. During the analysis of crop rhizosphere soil microbial diversity, higher values of Chao1, ACE, and Shannon indices, as well as lower values of Simpson index, reflected greater microbial diversity in the samples [13, 32]. Table 3 presents trends in the diversity and richness of bacterial, fungi, and archaeal in yellow and brown soil following the cultivation of Pseudostellaria heterophylla. In the aftermath of Pseudostellaria heterophylla cultivation, both yellow and brown soil exhibited a notable augmentation in bacterial richness, as reflected by ACE and Chao1 indices. A similar enhancement in fungal richness was observed in yellow soil. On the contrary, the cultivation of Pseudostellaria heterophylla led to a meaningful decrease in the ACE, Chao, and Shannon indices of archaea in yellow soil, though the Simpson index rose. These outcomes hint that Pseudostellaria heterophylla cultivation could amplify the α-diversity of bacterial and fungal in yellow soil, while concurrently diminishing the α-diversity of archaea. However, the effect on diversity within brown soil was comparatively mild. In summary, these data suggest that cultivation of Pseudostellaria heterophylla can impact soil microbial abundance and diversity, with varied effects across different soil types and microbial groups. These findings may offer insights into the ecological impact of Pseudostellaria heterophylla on soil ecosystems and provide guidance for optimizing soil microbial environments through adaptive cultivation strategies.
The predominant phylum exhibited variations among the diverse microbial communities across distinct soil types (Fig. 4). The prominent bacterial phylum identified were Acidobacteria, Actinobacteria, Chloroflexi, and Proteobacteria, collectively constituting over 70% of the total bacterial sequences. In both yellow and brown soils, a noteworthy rise in the abundance of Proteobacteria were observed compared to the control soils. In brown soil, the relative abundance of Acidobacteriota decreased significantly after Pseudostellaria heterophylla planting, while the relative abundance of Gemmatimonadota increased significantly. In yellow soil, we also noticed a marked increase in the abundance of Bacteroidetes. For Archaea, Thermoplasmatota and Crenarchaeota were the most abundant groups, comprising approximately more than 98% of the classified phylum Archaea in two treatments. No obvious differences in the abundance of Thermoplasmatota and Crenarchaeota were observed after planting Pseudostellaria heterophylla compared to the control soil for either yellow or brown soil. The most abundant among the known fungal phyla in both yellow and brown soils were Ascomycota, Mucoromycota, and Basidiomycota, accounting for over 80% of the detected fungal phyla. In the brown soil, the abundance of Ascomycta increased significantly after planting Pseudostellaria heterophylla compared to the control soil, while in the yellow soil, its abundance decreased significantly. The yellow soil exhibited a significant increase in the abundance of Mucoromycota. These findings imply that there are variations in the microbial response mechanisms to Pseudostellaria heterophylla cultivation in different soil types. The response of soil microbial communities to soil types varies between phyla or genera due to significant differences in the physicochemical properties of soils in different soil types [14]. Our study suggests that crops in different soil types may have different selections for microbial populations, which is consistent with previous studies [1, 19].
The microbial community structure of soil samples was analyzed at the class level. Circus plots show the distribution of relationships between microbial classes in different soil types at class level (Fig. 4b). It was found that Acidobacteriia and Alphaproteobacteria were the main bacterial classes. In the yellow soil, the relative abundance of these two classes increased from 19.21 and 18.55% to 21.10 and 29.56%, respectively, after the cultivation of Pseudostellaria heterophylla. However, in the brown soils, the relative abundance of Acidobacteriia and Alphaproteobacteria decreased from 35.22 and 29.10% to 25.89 and 23.14%. Sordariomycetes was the main fungal class, and in the yellow soil, its relative abundance decreased from 36.00 to 28.25%, due to the planting of Pseudostellaria heterophylla. In contrast, in brown soils, the relative abundance of Sordariomycetes increased from 13.24 to 23.50%. Incertae_Sedis was the second largest fungal class, and in these two types of soil, the relative abundance of Incertae_Sedis increased to varying degrees compared to the control soil. Nitrososphaeria was the most abundant microorganism in the analysis at the class level of archaeal microorganisms. There was no change in its relative abundance after cultivation of Pseudostellaria heterophylla in both yellow and brown soils. This result suggests to us that Nitrososphaeria may not be the most important factor driving the process for the continuous cropping obstacle of Pseudostellaria heterophylla. Class-level analyses indicated that cultivating Pseudostellaria heterophylla had a significant impact on the microbial community structure of both yellow and brown soils, potentially contributing to soil acidification. These results suggest that there are differences in microbial response to Pseudostellaria heterophylla cultivation in different soil types. Given the varying soil environments in different production areas of Pseudostellaria heterophylla, it is essential to consider the distinct soil microbiota structure in these areas. This information can be used to improve soil microbial environments based on local conditions, ultimately aiding in the efficient use of soils in various Pseudostellaria heterophylla main production areas. To investigate potential interactions between phenolic compounds and soil microbial communities, we constructed a microbial-phenolic acid co-occurrence network at the genus level (Fig. 5) [18, 31, 41]. In the bacterio-phenolic acid co-occurrence network, we could observe that Bradyrhizobium was a crucial node, and vanillic acid and benzoic acid were located close to it, suggesting that Bradyrhizobium might have been more susceptible to regulation by vanillic acid and benzoic acid. In the fungal communities, Calcarisporiella and Boothiomyces have a high degree of co-occurrences with phenolic acid components, and vanillin and vanillic acid may be potential regulators. Interestingly, at the genus level, the archaea-phenolic acid symbiotic network shows a more complex symbiotic relationship compared to the fungi, with Methanocella and Nitrosarchaeum likely to be the main players in archaeal microorganisms responding to the regulation of phenolic acid-like components. These findings contribute to the understanding of the complex relationships between phenolic components, microbial communities, and their interactions in the soil ecosystem.
Correlation and co-occurrence network analysis between microorganisms and phenolic acids. (a) Heatmap of correlation analysis between phenolic acids and bacteria, fungi, and archaea at the phylum level. (b–d) Co-occurrence network map at the genus level. Larger circles for nodes represent higher degree values, indicating these nodes have more connections with others in the network.
In order to examine the potential associations between phenolic acid compounds and the microbial community structure, we conducted an analysis on the impact of these compounds on the composition of bacteria, fungi, and archaea at the phylum level in rhizosphere soil of Pseudostellaria heterophylla. The correlations were visualized through a heatmap/. Proteobacteria showed positive correlations with benzoic and gallic acid, whereas Planctomycetes showed negative correlations with gallic acid. Cyanobacteria were negatively correlated with 4-hydroxybenzoic acid, vanillic acid, and vanillin. For the fungal microbiomes, Cryptomycota showed positive correlations with most phenolic acids. Most archaea microorganisms seemed to show negative correlations with phenolic compounds, but no significant findings were observed. The results displayed that the microbial community in rhizosphere soil of Pseudostellaria heterophylla was influenced by soil phenolic concentrations, with different microorganisms showing varying responses to the influence of phenolic acids. This observation highlights the important role that phenolic components play in shaping the microbial community structure within the rhizosphere soil of Pseudostellaria heterophylla, and the differences in the responses of different microorganisms could have been due to their specific physiological and metabolic characteristics. Further research was needed to understand the underlying mechanisms and the ecological implications of these findings.
CONCLUSIONS
Cultivation of Pseudostellaria heterophylla, whether on yellow or brown soils, can lead to increased levels of phenolic metabolites in the soil of the rhizosphere. However, the extent of the increase varies for each type of phenolic acid in different soils. Cultivation of Pseudostellaria heterophylla can increase the α-diversity of bacteria and fungi in yellow soils, while decreasing the α-diversity of archaea. In brown soil, it significantly increases archaeal diversity. Therefore, when developing strategies to reduce the barriers of successive monocultures of medicinal plants, the different responses of Pseudostelariae heterophylla growth in different soil types should be considered. For yellow soils, attention should mainly focus on changes in bacterial and fungal microorganisms. For brown soils, more attention should be paid to archaea. This study provides new insights that the variability in the response of different soil types to continuous cropping obstacle should be taken into account when developing strategies for reducing continuous cropping obstacles in Pseudostellaria heterophylla
REFERENCES
R. Bai, J. T. Wang, Y. Deng, J. Z. He, K. Feng, and L. M. Zhang, “Microbial community and functional structure significantly varied among distinct types of paddy soils but responded differently along gradients of soil depth layers,” Front. Microbiol. 8, (2017). https://doi.org/10.3389/fmicb.2017.00945
L. Bao, Y. Liu, Y. Ding, J. Shang, Y. Wei, Y. Tan, and F. Zi, “Interactions between phenolic acids and microorganisms in rhizospheric soil from continuous cropping of panax notoginseng,” Front. Microbiol. 13, 791603 (2022). https://doi.org/10.3389/fmicb.2022.791603
J. Chen, W. Pang, Y. Kan, L. Zhao, Z. He, W. Shi, B. Yan, H. Chen, and J. Hu, “Structure of a pectic polysaccharide from Pseudostellaria heterophylla and stimulating insulin secretion of INS-1 cell and distributing in rats by oral,” Int. J. Biol. Macromol. 106, 456–463(2018). https://doi.org/10.1016/j.ijbiomac.2017.08.034
I. D. Grodnitskaya, O. E. Pashkeeva, V. V. Startsev, and A. A. Dymov, “Respiratory activity and biodiversity of microbiomes in podzolic soils of post-pyrogenic spruce forests in the Krasnoyarsk krai and Komi Republic,” Eurasian Soil Sci. 56 (6), 793–806 (2023). https://doi.org/10.1134/S1064229323600379
W. Dong, E. Liu, C. Yan, H. Zhang, and Y. Zhang, “Changes in the composition and diversity of topsoil bacterial, archaeal and fungal communities after 22 years conventional and no-tillage managements in Northern China,” Arch. Agron. Soil Sci. 63 (10), 1369–1381 (2017). https://doi.org/10.1080/03650340.2017.1281392
J. Gu, G. Zou, S. Su, S. Li, W. Liu, H. Zhao, L. Liu, L. Jin, Y. Tian, X. Zhang, Y. Wang, T. Zhao, L. Du, and D. Wei, “Effects of pH on available cadmium in calcareous soils and culture substrates,” Eurasian Soil Sci. 55 (12), 1714–1719 (2022). https://doi.org/10.1134/S1064229322601391
J. Hu, W. Pang, J. Chen, S. Bai, Z. Zheng, and X. Wu, “Hypoglycemic effect of polysaccharides with different molecular weight of Pseudostellaria heterophylla,” BMC Complementary Altern. Med. 13 (1), 267 (2013). https://doi.org/10.1186/1472-6882-13-267
C. Ji, J. Huang, X. Zhang, G. Yang, S. Xing, W. Fu, Z. Hao, B. Chen, and X. Zhang, “Response of soil fungal community to chromium contamination in agricultural soils with different physicochemical properties,” Sci. Total Environ. 879, 163244 (2023). https://doi.org/10.1016/j.scitotenv.2023.163244
Y. Kan, Y. Liu, Y. Huang, L. Zhao, C. Jiang, Y. Zhu, Z. Pang, J. Hu, W. Pang, and W. Lin, “The regulatory effects of Pseudostellaria heterophylla polysaccharide on immune function and gut flora in immunosuppressed mice,” Food Sci. Nutr. 10 (11), 3828–3841 (2022). https://doi.org/10.1002/fsn3.2979
Y. Li, L. Xu, P. Letuma, and W. Lin, “Metabolite profiling of rhizosphere soil of different allelopathic potential rice accessions,” BMC Plant Biol. 20 (1), 265 (2020). https://doi.org/10.1186/s12870-020-02465-6
P. Li, Y. Yan, C. Li, W. Tang, Z. Xiong, Y. Tian, K. Zhou, Z. Yi, Z. Zheng, Z. Rang, and J. Li, “Response of rice growth to soil microorganisms and soil properties in different soil types,” Agron. J. 115 (1), 197–207 (2023). https://doi.org/10.1002/agj2.21239
Y. Liu, Y. Kan, Y. Huang, C. Jiang, L. Zhao, J. Hu, and W. Pang, “Physicochemical characteristics and antidiabetic properties of the polysaccharides from Pseudostellaria heterophylla,” Molecules 27 (12), (2022). https://doi.org/10.3390/molecules27123719
X. Liu, Y. Shi, L. Kong, L. Tong, H. Cao, H. Zhou, and Y. Lv, “Long-term application of bio-compost increased soil microbial community diversity and altered its composition and network,” Microorganisms 10 (2), 462 (2022). https://doi.org/10.3390/microorganisms10020462
Y. Lu, W. Zhang, Y. Zhang, S. Wu, M. Ma, X. Peng, Z. Zeng, and D. Zeng, “Metabolite identification of isopropoxy benzene guanidine in rat liver microsomes by using UHPLC-Q-TOF-MS/MS,” Int. J. Mol. Sci. 24 (8), 7313 (2023). https://doi.org/10.3390/ijms24087313
C. A. Medriano, A. Chan, R. De Sotto, and S. Bae, “Different types of land use influence soil physiochemical properties, the abundance of nitrifying bacteria, and microbial interactions in tropical urban soil,” Sci. Total Environ. 869, (2023). https://doi.org/10.1016/j.scitotenv.2023.161722
L. Meng, Z. Xia, J. Lv, G. Liu, Y. Tan, and Q. Li, “Extraction and GC-MS analysis of phenolic acids in rhizosphere soil of Pinellia ternate,” J. Radiat. Res. Appl. Sci. 15 (3), 40–45 (2022). https://doi.org/10.1016/j.jrras.2022.05.015
K. Nadarajah and N. S. N. Abdul Rahman, “The microbial connection to sustainable agriculture,” Plants (Basel) 12 (12), (2023). https://doi.org/10.3390/plants12122307
S. Niraula, M. Rose, and W. S. Chang, “Microbial co-occurrence network in the rhizosphere microbiome: its association with physicochemical properties and soybean yield at a regional scale,” J. Microbiol. 60 (10), 986–997 (2022). https://doi.org/10.1007/s12275-022-2363-x
F. Oehl, E. Laczko, A. Bogenrieder, K. Stahr, R. Bösch, M. van der Heijden, and E. Sieverding, “Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities,” Soil Biol. Biochem. 42 (5), 724–738 (2010). https://doi.org/10.1016/j.soilbio.2010.01.006
M. Oh, S. Park, H. Kim, G. J. Choi, and S. H. Kim, “Application of UPLC-QTOF-MS based untargeted metabolomics in identification of metabolites induced in pathogen-infected rice,” Plants (Basel) 10 (2), (2021). https://doi.org/10.3390/plants10020213
X. Qin, H. Wu, J. Chen, L. Wu, S. Lin, M. U. Khan, M. R. Boorboori, and W. Lin, “Transcriptome analysis of Pseudostellaria heterophylla in response to the infection of pathogenic Fusarium oxysporum,” BMC Plant Biol. 17 (1), 155 (2017). https://doi.org/10.1186/s12870-017-1106-3
X. H. Qu and J. G. Wang, “Effect of amendments with different phenolic acids on soil microbial biomass, activity, and community diversity,” Appl. Soil. Ecol. 39 (2), 172–179 (2008). https://doi.org/10.1016/j.apsoil.2007.12.007
F. Rasche and G. Cadisch, “The molecular microbial perspective of organic matter turnover and nutrient cycling in tropical agroecosystems—what do we know?,” Biol. Fert. Soils 49 (3), 251–262 (2013). https://doi.org/10.1007/s00374-013-0775-9
J. M. Reichert, B. Morales, E. M. Lima, F. de Bastos, C. A. S. Morales, and E. F. de Araújo, “Soil morphological, physical and chemical properties affecting Eucalyptus spp. productivity on Entisols and Ultisols,” Soil Tillage Res. 226, 105563 (2023). https://doi.org/10.1016/j.still.2022.105563
L. Su, H. Li, X. Sun, K. Wang, X. Shu, W. Gao, Y. Liu, E.E. Kuramae, B. Shen, and R. Zhang, “Identification of general features in soil fungal communities modulated by phenolic acids,” Appl. Soil Ecol. 189, 104909 (2023). https://doi.org/10.1016/j.apsoil.2023.104909
B. Wang, Y. Lin, W. Yu, Q. Xia, A. Ali, F. Wei, C. Dai, J. Zhang, Z. Cai, and J. Zhao, “The loss of microbial autotoxin degradation functions is associated with the decline of beneficial bacterial agents induced by phenolic acids,” Microbiol. Spectrum, (2023). https://doi.org/10.1128/spectrum.03380-22
J. Wang, Q. Wei, W. Wang, H. Hu, Y. Yan, Y. Wang, Y. Li, Y. Jiang, G. Wu, T. Hu, and C. Bao, “Understanding the nutraceutical diversity through a comparative analysis of the taproot metabolomes of different edible radish types via UHPLC–Q–TOF–MS,” Food Chem. 403, 134469 (2023). https://doi.org/10.1016/j.foodchem.2022.134469
T. G. Weldmichael, E. Michéli, H. Fodor, and B. Simon, “The influence of depth on soil chemical properties and microbial respiration in the upper soil horizons,” Eurasian Soil Sci. 53 (6), 780–786 (2020). https://doi.org/10.1134/S1064229320060137
L. Wu, Z. Li, J. Li, M. A. Khan, W. Huang, Z. Zhang, and W. Lin, “Assessment of shifts in microbial community structure and catabolic diversity in response to Rehmannia glutinosa monoculture,” Appl. Soil Ecol. 67, 1–9 (2013). https://doi.org/10.1016/j.apsoil.2013.02.008
L. Wu, B. Yang, M. Li, J. Chen, Z. Xiao, H. Wu, Q. Tong, X. Luo, and W. Lin, “Modification of rhizosphere bacterial community structure and functional potentials to control Pseudostellaria heterophylla replant disease,” Plant Dis. 104 (1), 25–34 (2020). https://doi.org/10.1094/PDIS-04-19-0833-RE
H. Wu, W. Yan, H. Wu, J. Zhang, Z. Zhang, Z. Zhang, C. Rensing, and W. Lin, “Consecutive monoculture regimes differently affected the diversity of the rhizosphere soil viral community and accumulated soil-borne plant viruses,” Agric. Ecosyst. Environ. 337, 108076 (2022). https://doi.org/10.1016/j.agee.2022.108076
J. Xing, X. Wang, C. Hu, L. Wang, Z. Xu, X. He, Z. Wang, P. Zhao, and Q. Liu, “Effects of residual mulching films with different mulching years on the diversity of soil microbial communities in typical regions,” Heliyon 8 (12), e12180(2022). https://doi.org/10.1016/j.heliyon.2022.e12180
X. Yang, H. W. Hu, G. W. Yang, Z. L. Cui, and Y. L. Chen, “Crop rotational diversity enhances soil microbiome network complexity and multifunctionality,” Geoderma 436, 116562 (2023). https://doi.org/10.1016/j.geoderma.2023.116562
Q. S. Yuan, L. Wang, H. Wang, X. Wang, W. Jiang, X. Ou, C. Xiao, Y. Gao, J. Xu, Y. Yang, X. Cui, L. Guo, L. Huang, and T. Zhou, “Pathogen-mediated assembly of plant-beneficial bacteria to alleviate Fusarium wilt in Pseudostellaria heterophylla,” Front. Microbiol. 13, 842372 (2022). https://doi.org/10.3389/fmicb.2022.842372
L. Zhang, Z. Guo, H. Gao, X. Peng, Y. Li, S. Sun, J. K. Lee, and W. Lin, “Interaction of Pseudostellaria heterophylla with quorum sensing and quorum quenching bacteria mediated by root exudates in a consecutive monoculture system,” J. Microbiol. Biotechnol. 26 (12), 2159–2170 (2016). https://doi.org/10.4014/jmb.1607.07073
J. Zhou, L. Qi, and P. Li, “Quality control of Chinese herbal medicines with chromatographic fingerprint,” Chin. J. Chromatogr. 26 (2), 153–159 (2008). https://doi.org/10.1016/S1872-2059(08)60011-5
Y. Zhao, L. Wu, L. Chu, Y. Yang, Z. Li, S. Azeem, Z. Zhang, C. Fang, and W. Lin, “Interaction of Pseudostellaria heterophylla with Fusarium oxysporum f.sp. heterophylla mediated by its root exudates in a consecutive monoculture system,” Sci. Rep. 5, 8197 (2015). https://doi.org/10.1038/srep08197
W. Zhao, Y. Li, C. Yang, Y. Yang, and Y. Hu, “Rhizosphere microbial community and metabolites of susceptible and resistant tobacco cultivars to bacterial wilt,” J. Microbiol. 61 (4), 389–402 (2023). https://doi.org/10.1007/s12275-023-00012-0
Q. Zhao, W. Chen, L. Wu, B. Wang, Y. Wu, H. Chen, Y. Bai, and D. Chen, “Nitrogen enrichment amplifies the effects of litter treatments on soil microorganisms and ecosystem functions in grazed and non-grazed grasslands,” Appl. Soil Ecol. 186, 104851 (2023). https://doi.org/10.1016/j.apsoil.2023.104851
X. Zhou, G. Yu, and F. Wu, “Soil phenolics in a continuously mono-cropped cucumber (Cucumis sativus L.) system and their effects on cucumber seedling growth and soil microbial communities,” Eur. J. Soil Sci. 63 (3), 332–340 (2012). https://doi.org/10.1111/j.1365-2389.2012.01442.x
P. Zhu, W. Liu, Z. Sun, X. Bai, J. Song, N. Wu, and Y. Hou, “Soil bacterial community response to fire varies with slope aspect at Zhenshan Mountain, East China,” Eurasian Soil Sci. 56 (5), 599–610 (2023). https://doi.org/10.1134/S1064229322602104
ACKNOWLEDGMENTS
We would like to thank Dr. Ping Tong (Fuzhou University) for his help with soil metabolite analysis and Dr. Ziqin Pang (Fujian Agriculture and Forestry University) for his help with the data visualization of the co-occurrence network analysis.
Funding
This work was funded by the Fundamental Research Project for Commonweal Scientific Institutes in Fujian Province, project no. 2020R1003004, the Major Special Project of Fujian Provincial Science and Technology Department, project no. 2022NZ029015.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally to this work.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, Y., Wu, D., Kan, Y. et al. Response of Soil Microorganisms and Phenolic to Pseudostelariae heterophylla Cultivation in Different Soil Types. Eurasian Soil Sc. 57, 446–459 (2024). https://doi.org/10.1134/S1064229323602640
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229323602640