Abstract
The transformation of technogenic Cu and Zn compounds in technogenically transformed soils (Spolic Technosols) with high and very high concentrations of metals formed at the site of a natural tailings pond in the floodplain of the Seversky Donets River, the main tributary of the Don River (Rostov oblast, Russia) has been studied. The Technosols are compared to an unpolluted meadow-chernozemic soil (Fluvisol) outside the impact zone. The state of Cu and Zn is assessed using three sequential extraction schemes—Miller’s, Tessier’s, and BCR, as well as synchrotron X-ray powder diffraction (XRD) and analysis of synchrotron X-ray absorption spectrometry (XAFS) spectra. It is shown that the distribution of metals in soil is largely related to their properties, such as electronegativity, hydrolyzability, and softness parameter. As is observed, Cu mainly concentrates in the residual fraction (to 42%) and in the fraction associated with organic matter (up to 27%). The mobility of Zn in the studied soils is higher than that of Cu. Its main part (up to 56%) is in the residual fraction and the fraction associated with Fe and Mn oxides (up to 48%), especially with Fe(III) crystalline compounds. The combination of a three-stage BCR scheme with XAFS and XRD techniques is used for the first time. Most of the peaks in diffraction patterns of soil samples after the first and second extraction stages correspond to the authigenic sulfur-containing minerals, namely, wurtzite (ZnS with a hexagonal structure), sphalerite (cubic ZnS), covellite (CuS), and bornite (Cu5FeS4). Wurtzite is present in the exchangeable and reducible fractions. These fractions also contain chalcocite (Cu2S). Sulfides are most abundant in soil sample after extraction of the oxidizable fraction, while phyllosilicates are prevalent in the sample after extraction of the reducible fraction. X-ray absorption spectroscopy demonstrates molecular structural changes in the Zn and Cu compounds in heavily polluted soils, suggesting the transformation of metals under different environmental conditions, which is important for assessment of the soil protective function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, a rapid growth in the industrial use of nonferrous metals inevitably leads to environmental pollution and, mainly, soil pollution, by heavy metal (HM) compounds of a technogenic origin. The bioavailability of HMs exceeds the homeostatic control of a living organism [36]. Correspondingly, HMs become hazardous when they enter the food web. Some HMs, for example, Cu and Zn, are most important trace elements but, concurrently, are highly toxic to animals and humans, belonging to hazard classes II and I.
Metals and metalloids play a key role in the function and resistance of soil ecosystems. Scientists and experts has reached the understanding that the HM transformation by soils, leading to formation of various metal compounds, is a key factor that regulates their behavior and functions in the “soil–plant” system and, consequently, the possibility for HMs to enter the food chain [44]. In the undisturbed natural soils with the age of several hundred to several thousand years (depending on the formation conditions), the HMs that originated from the initial rock are transformed very slowly [1, 32]. The ratio of metal compounds in soil in the absence of pollution reflects the stability of soil-forming processes.
However, the advance in science and technology causes an increase in HM concentrations in environment and, especially, in the soils of impact areas, to the levels that considerably exceed the threshold limit concentrations [13]. The transformation of technogenic HM compounds in the soils of this type is considerably accelerated because of thermodynamic imbalance resulting from oxidation, carbonization, sedimentation of secondary minerals, and other factors [34, 36].
Correspondingly, it is important to have an idea of how the technogenic metal compounds have been transformed depending on the soil-forming conditions, soil properties, and specific pollution features.
Sequential selective extractions are widely used to study the HM compounds in polluted soils. Although differing in detail, the extraction-based techniques of chemical analysis are basically similar. These methods rely on the use of extracting agents that bring into solution the metal compounds presumably hold by solid-phase soil components via different mechanisms and with different strengths [28]. The research into HM absorption by different soil components, such as organic matter, clay materials, Fe–Mn hydroxides, and carbonates, is most relevant.
When working with a complex polydisperse heterogeneous system, such as soil, almost all used schemes of sequential selective extractions have rather serious shortcomings. In particular, this includes insufficiently high selectivity of the used extracting agents, especially in the case of extraction of weakly bound species; incomplete extraction of HMs from target carrier phases; and re-absorption and re-precipitation [9, 21, 22, 33, 38]. In addition, the stability of HM binding to the surface of soil particles and, correspondingly, their extractability, to a considerable degree depend on the composition of the liquid phase, metal properties, and the degree of filling of the sorbent’s surface (the level of pollution) [7, 32]. During sequential extraction of pollutants from soil, the system in general changes in an uncontrollable manner, which naturally influences the result of the study [28, 33]. The difficulties in detecting the species of metal compounds are especially pronounced for the highly dynamic technogenic soil ecosystems. These methods are usually combined with other diagnostic methods in order to increase the information content [25, 27, 33]. Synchrotron X-ray absorption spectroscopy is currently among the most promising methods for nondestructive diagnostics of the local atomic and electron structures of materials without a long-range order in atom arrangement. The spectroscopy hardware is constantly improved and the number of studies applying this method is increasing [23, 40]. Correspondingly, it is purposeful to study the HM compounds using the extraction fractionation and X-ray absorption spectroscopy.
The goal of this work was to study the states of Zn and Cu cations in technogenically transformed soils using sequential selective extractions and X-ray synchrotron radiation.
OBJECTS AND METHODS
The examined area is an impact site in the floodplain of the Seversky Donets River (a main tributary of the Don River, southern Russia), which was used in the 1950s–1990s as the basin for wastewater disposal of a chemical plant. It is a dried oxbow of Atamanskoe Lake (48°21′00″ N, 40°14′31″ E) and is now a secondary source of environmental pollution [27]. A geochemical grid of 100 monitoring sites was constructed for studying this area. The examined area amounted to approximately 12 ha. Soil was sampled in the dried lake to a depth of 0–20 cm according to the state standard GOST 28168-89.
The soil in this territory has been formed for several tens of years from the outcrops of the polluted bottom sediments during shallowing and drying of the lake. The studied technogenically transformed soils are technogenically transformed soil(Spolic Technosols). Two sites in the dried lake were selected for monitoring based on the lithochemical testing, one with a high level of anthropogenic pollution (Technosol-1) and the other, with an abnormally high level (Technosol-2). Technosol -1 resides in the southern part of Atamanskoe Lake on the left bank of the Seversky Donets River, 500 m to the north of the water edge, and Technosol-2, in the southern end of the lake where the tube for the discharge of chemical facility waste was presumably located. A meadow-chernozem soil (Fluvisol) in the monitoring site at a distance of 1200 m from the lake in the floodplain margin on the right bank of the Seversky Donets River 100 m to the south of the water edge was used as the reference
The physical and chemical characteristics of the studied soils were analyzed using conventional standard methods, including pH by potentiometry at a soil to water ratio of 1 : 2.5; content of organic carbon (Corg) by titrimetry (bichromate oxidation according to Tyurin) [3]; cation exchange capacity according to Shaimukhametov [12]; content of carbonates by complexometry according to Kudrin [3]; and particle-size distribution by pipetting with pyrophosphate treatment of the samples [2]. The elemental composition of soils was determined by X-ray fluorescence in a Spektroskan-MAKS-GV (Spektron, Russia) spectrometer.
Numerous schemes for HM sequential fractionation in soils have been developed [6–8]. In most cases, original “author’s” methods for HM extraction are utilized. The most widespread is Tessier’s technique, which has so far 7700 citations, and BCR technique, recommended by the European Community Bureau of Reference and modified by adding the residual fraction [31, 42]. In this work, we used Tessier’s technique, modified BCR scheme, and the method by Miller et al. [26], which make it possible to study the patterns of HM transformation on Fe and Mn oxides in more detail (Table 1). The concentrations of elements in each fraction are given in absolute and relative units, allowing the Cu and Zn shares in each fraction to be assessed with regard to the total amount of extracted metals. Synchrotron X-ray absorption spectrometry (XAFS) and X-ray powder diffraction (XRD) of the sample after each stage of sequential extraction using the international BCR scheme [40] were used to identify the phases retaining Cu2+ and Zn2+ in technogenically disturbed soil. The nondestructive XAFS techniques, such as extended X-ray absorption fine structure (EXAFS) and X-ray absorption near-edge structure (XANES) have been used. The radial distribution functions around metal atoms were obtained with the help of the Fourier transform of k weighted EXAFS functions in the range of photoelectron wave numbers of 2.6–12.5 Å–1. Because of the constraints associated with the sensitivity of experimental tools for measuring XAFS spectra, we used the soil with an abnormally high pollution level (Technosol-2).
The EXAFS (long-range) and XANES (near edge) experimental data (Zn and Cu K-edge, 9659 eV) were obtained at the Structural Material Science station on the 1.3b channel at the Kurchatov Center for Synchrotron Radiation (National Research Center Kurchatov Institute). A 1.7 Tl bend magnet of the Siberia-2 storage ring was used as the source of synchrotron radiation. The electron beam energy for generation of synchrotron radiation was 2.5 GeV and average current, 60–70 mA.
The experimental EXAFS spectra were routinely processed using Fourier filtration followed by fitting of the varied parameters of the local atomic structure with the help of FEFFIT [35] and Viper software.
The XRD data were analyzed in a Belok/XSA station; the wavelength of the used monochromatic radiation was 0.8 Å (photon energy, 15 498 eV). A sample was placed into a cryoloop of 300 µm. Diffraction patterns were recorded with a 2D Rayonix SX165 detector. After removal of the background and normalization of diffraction patterns, relative intensities, Bragg angles, and the corresponding interlayer distances were determined for all diffraction peaks; the peaks were identified using crystallography databases; and the mineral components remaining in the solid phase after each selective extraction were detected. Three diffraction patterns were recorded for each sample using different aliquots and were compared to assess the homogeneity of samples.
RESULTS AND DISCUSSION
Physicochemical characteristics of soils. Analysis of the physicochemical characteristics of soils (Table 2) has shown a high Corg content (4.3 ± 0.3%) at a neutral pH (7.5 ± 0.2) in the unpolluted meadow soil. The Corg content in Technosols varies in the range of 2.4–4.4% and pH, 7.7–8.2 (weakly alkaline). Ca2+ is prevalent in the exchange complex. The studied soils have a heavy loamy particle-size distribution. The content of physical clay (particles <0.01 mm) amounts to 48.4–52.1% and of clay (<0.001), 26.0–33.0%. The contents of these fractions in meadow soil are close. The bulk HM content in soils of the monitoring sites is rather different and considerably exceeds the threshold concentrations of the studied elements according to Sanitary Regulation and Standard SanPiN 2.1.3684-21. Note that Zn is the major pollutant in the studied soils: its concentration in Technosol-2 is over 6.2 wt %.
Assessment of Cu and Zn state in Technosols according to fractionation results. The data on the redistribution of exogenous Cu and Zn between the fractions isolated using different techniques are listed in Table 3.
In order to reveal the general patterns in the redistribution of metals between fractions in Technosols, the data on the metal contents in the fractions obtained using different fractionation schemes were compared to each other and to the meadow soil. As is shown, the contents of metals in all fractions of the meadow soil except for the residual fraction (to 61 and 85% of the sum of the fractions) are very low and are observed in all fractionation schemes. A low mobility of Zn and Cu and their prevalence in the residual fraction confirm that the amount of these metals being technogenic is rather small.
The Cu and Zn accumulation in Technosols is higher as compared with the meadow soil. The differences in the fractions associated with organic matter, Fe and Mn sesquioxides are especially pronounced. The main distinctions in the results of HM fractionation for Technosols using different techniques consist in different extraction abilities of the used reagents. Of the used methods, Miller’s scheme utilizes “weaker” extracting agents for isolation of HMs associated with organic matter and the Fe and Mn oxides. Correspondingly, the content of metals in these fractions obtained using Tessier’s, and BCR schemes is somewhat higher as compared with that obtained according to Miller’s scheme (Fig. 1). On the other hand, the use of Miller’s scheme has clarified the role of amorphous and crystalline Fe oxides in the retention of metals in Technosols. In the interaction with Zn, the crystalline Fe oxides display a higher affinity for this element as compared with the amorphous ones, which is determined by a long-term pollution of this area since Fe oxides take a long time to crystallize in soil [14].
The data for the fractions associated with the Mn and Fe oxides in Miller’s scheme were pooled. As it emerged in this approach, the percent rates of Cu and Zn in the fractions associated with Fe and Mn oxides in Miller’s scheme insignificantly differ from the values determined using Tessier’s and BCR schemes and change in the same direction depending on the sum of fractions in Technosols. In particular, the share of Cu in the fractions associated with Fe and Mn oxides in Miller’s, Tessier’s, and BCR schemes in Technosol-1 is 13.05, 17.6, and 17.4%, respectively, and in Technosol-2, 8.02, 9.2, and 8.2%. As for Zn, its share in the fractions associated with Fe and Mn oxides in Miller’s, Tessier’s, and BCR schemes in Technosol-1 is 24.4, 27.0, and 24.0%, respectively, and in Technosol-2, 34.1, 38.1, and 48.3% (Fig. 1). The least amount of metals passes into the water-soluble fraction (≤0.3% Cu and ≤2.2% Zn) and the largest amount, into the residual fraction and the fractions associated with Fe and Mn oxides and organic matter. The share of Cu in the fractions extracted from polluted soils increases in the following order: water-soluble \( \ll \) exchangeable < associated with carbonates < associated with different Fe and Mn oxides compounds < associated with organic matter \( \ll \) residual. The exceptions are water-soluble and residual fractions. The Cu content in water-soluble fractions differs insignificantly. The share of Cu in the residual fraction of Technosol-2 is larger as compared with the corresponding fraction of Technosol-1. Thus, Cu is Technosol-1 is in general weaker retained by the carrier phases as compared with Technosol-2. A considerable part of the metal is redistributed between other, less stable carrier phases. A larger share of Cu associated with the residual fraction of Technosol-2 as compared with Technosol-1 confirms this pattern.
The share of Zn in the fractions of Technosol-1 increases in the following order: water-soluble \( \ll \) associated with organic matter < associated with carbonates < exchangeable < associated with different species of Fe and Mn oxides < residual; as for Technosol-2, the order is the following: water-soluble < associated with organic matter < associated with carbonates ≤ exchangeable < residual < associated with different compounds of Fe and Mn oxides. The change in the order of Zn distribution between the extracted fractions as compared with Cu, first and foremost, suggests a change in the character of interaction between the absorbed metals and carrier phases, which results from the changes in the chemical composition of these fractions as well as individual properties of metals. The Cu content in Technosol-1 is 3.5-fold higher as compared with Technosol-2 and the Zn content is 26-fold lower. Although the studied metals mainly concentrate in the residual fraction, a considerable amount of Cu also accumulates in the fraction associated with soil organic matter (25.3%), whereas Zn to a higher degree concentrates in the fraction associated with Fe and Mn oxides [11].
The share of Cu in the residual fraction of Fluvisol is 84.9% and in the remaining fractions, 15.1%; of them, 10.1% are present in the fraction associated with organic matter. This is smaller as compared with Technosols but considerably larger as compared with the fractions extracted from carbonates and oxides of Fe and Mn. The Zn distribution in the reference soil is more uniform. The largest amount of Zn is extracted from the residual fraction (61.3%) versus 38.7%, extracted from the remaining fractions; of them, 16.7% is extracted from the fraction associated with Fe and Mn oxides. The order of Cu distribution in the reference soil is the same as in Technosol-1 and Technosol-2 and of Zn, the same as in Technosol-1. Thus, Cu is much more stably sorbed than Zn in both polluted and reference soils. Correspondingly, Zn is more mobile as well as has higher migration ability and availability to plants. Soil organic matter plays an important role in Cu absorption by Technosols (up to 27% of the sum of fractions); the content of Cu is determined by physicochemical, biological, and environmental conditions, which limit the rate of its destruction [5]. Zn more intensively accumulates in Fe–Mn (hydr)oxides (to 48%), complying with the data of many studies [17, 38].
Individual properties of Cu and Zn significantly influence the pattern of their interaction with soils and, consequently, the fractional composition of these metals [10]. Several approaches have been used to explain this phenomenon. Some researchers link this to the hydrolytic properties of metals. In particular, the first hydrolysis constant for Cu (pK1 ~ 7.3–8.0) exceeds that for Zn (pK1 ~ 9.0–9.4). Correspondingly, Cu2+ ions will be more stably sorbed by soil as compared with Zn2+ [9]. In addition, prevalent in the soils with high pH are the partially hydrolyzed metal species (MOH+), which are stronger sorbed by soils as compared with the bivalent cations [19]. This mechanism also plays an important role in the HM absorption, especially, by alkaline soils.
Evans et al. [16] linked the soil affinity for metals to their electronegativity. Since Cu is more electronegative than Zn (2 > 1.6, respectively), the sorption of Cu by soils is more preferable than the sorption of Zn. According to McBride [24], electronegativity is an important factor determining the HM capability of chemosorption; correspondingly, he ordered the elements as Cu > Ni > Co > Pb > Cd > Zn > Mg > Sr. Misono et al. [29] proposed a two-parametric schedule for assessing the acidity of metal ions according to Lewis. One parameter is associated with electronegativity and the other one, calculated basing on the ion charge and radius, characterizes the “softness” of the bond, that is, the ability to form a covalent π bond. The latter is twofold higher for Cu as compared with Zn; that is why, the soil affinity for Cu is higher than that for Zn [36].
The stabilization of HMs in crystal lattices of clay minerals and the opposite process of their release are rather slow because of a complex multistage character of the process. Correspondingly, Cu and Zn have accumulated in the residual fractions of the studied soils over 60 years of pollution. On the other hand, the trend of a decrease in the share of residual fraction from 57–62 to 42–49% for Cu and from 47–56 to 37–28% for Zn is observed with an increase in the total concentration of metals in soil. Concurrently, the share of more mobile and thermodynamically less stable (exchangeable and associated with carbonates) fractions increases. In all cases, Zn is more mobile as compared with Cu. These patterns, namely, a relatively high mobility of Zn in technogenically disturbed soils, which makes this metal more mobile as compared with Cu, were reported in several papers [7, 18]. However, note that although the bulk Zn content in the studied soils is abnormally high (>62000 mg/kg), the relative Zn content in the exchangeable fraction and the fraction associated with carbonates is rather low (14–19% depending on the fractionation technique). A high content of organic matter, weakly alkaline pH, prevalence of highly dispersed clay and fine silty fractions in the studied soils (Table 1), and the absence of constant pollution source are the factors that determine this situation.
Thus, the formation patterns of Cu and Zn fractional composition in hydrogenic technogenically transformed soils observed using different schemes of sequential fractionation reveal both the general and specific features in the distribution of metals between fractions in Technosols associated with soil properties, character of pollution, and individual properties of metals. With an increase in the level of pollution, the share of HMs in the residual fraction decreases, while their share in more mobile compounds increases. In this process, the share of Zn in the first, more mobile fractions is always higher as compared with that of Cu. To a considerable degree, this is associated with the differences between these elements in the hydrolyzability, electronegativity, and softness parameter (according to Misono). The accumulation of Cu and Zn in the residual fraction of the reference soil suggests that a large part of the metals is stably bound to and stably fixed in the crystal lattices of aluminosilicates.
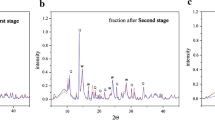
Assessment of Cu and Zn state in Technosols according to XRD data. Synchrotron X-ray diffraction patterns were recorded for Technosol-2 after each stage of BCR sequential extraction (Table 4). The most pronounced differences in the intensity of individual maximums are observed when comparing the diffraction patterns after the first extraction stage (removal of acid-soluble compounds). After the second extraction stage, the variations in the intensity of individual maximums are observable in diffraction patterns. After the third extraction stage, soil sample appears to be the most uniform in terms of mineral crystalline phases composition suggesting an almost complete coincidence of the diffraction patterns of all three replicates.
One of the prevalent components in soil samples after all fractionation stages is quartz. Most peaks present in the diffraction patterns of the soil samples after the first and second stages correspond to the authigenic sulfur-containing minerals, namely, wurtzite (ZnS with a hexagonal structure), sphalerite (cubic ZnS), covellite (CuS), and bornite (Cu5FeS4). Wurtzite is present in the exchangeable and reducible fractions. Sulfides are most abundant in soil samples after the first extraction stage, while phyllosilicates are prevalent in the samples after the extraction of reducible fraction. Montmorillonite is detectable after all fractionation stages. This is also true for rarely occurring anhydrite and wollastonite. Note that the intensities and positions of peaks depend on many factors, including the state of mineral components in the sample. Most likely, the soil chemical processing during sequential selective extractions, aimed at isolation of certain components, significantly influences the state of crystallites in the residue. A specific interaction of extracting agents with mineral and organic soil components has a strong and uncontrollable effect on the state of mineral components in the sample and on the overall sample. Presumably, this is the particular factor associated with the variations in intensities of individual maximums in diffraction patterns [4]. The positions of individual components in XRD spectra may be also explained by these processes.
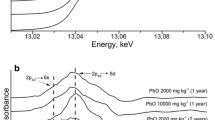
Assessment of Cu and Zn state in Technosols according to XAFS spectroscopy data. The sensitivity of the pre-edge region of XANES spectra can be used for preliminary qualitative analysis of the Cu and Zn surroundings in soil samples after each of three sequential extraction stages. The types of atoms forming the nearest surrounding of Cu atoms in Technosol samples after sequential extraction is determined via analysis of the Cu K-edge XANES spectra (Fig. 2a). Several copper-containing compounds with the closest coordination spheres of copper atoms formed by oxygen atoms (Cu(CH3COO)2, CuCO3, and CuSO4) as well as sulfur atoms (CuS and Cu2S) were used as references. The metal in CuSO4 is coordinated by four oxygen atoms with two short and two long Cu–O bonds (1.91 and 2.05 Å, respectively); the next coordination sphere has a radius of 2.37 Å and contains two additional oxygen atoms. Copper in Cu(CH3COO)2 also resides in tetrahedral oxygen structure with the length of Cu–O bond varying from 1.90 to 2.05 Å. The copper atoms in Cu2S are coordinated by three sulfur atoms and the closest Cu–S distance is 2.29 Å. The radius of the first coordination sphere in CuS, formed by three sulfur atoms, is 2.17 Å. A considerable difference in the position of absorption edge and the values of the main specific spectral features in the samples with Cu–S and Cu–O bonds make it possible to reliably distinguish between these types of Cu surrounding in soil samples after sequential selective extractions.
The Cu K-edge XANES spectra of Technosol samples after the first and second fractionation stages are very similar to the spectra of copper-containing compounds with sulfur (CuS and Cu2S), suggesting the presence of numerous Cu–S bonds. An additional confirmation is the pattern of radial distribution of atoms, assessable from FT (Fourier transformed) EXAFS spectra (Fig. 2b). The Cu K-edge XANES spectrum of the sample after the third stage has a pronounced maximum typical of the spectra of the reference compounds with oxygen surrounding of copper. The prevalence of Cu–O bonds in the fraction after the third stage is evidenced by the similarity in position of the absorption edge to the reference compounds.
Fitting of the spectra of the studied Technosol samples using a linear combination of reference spectra made it possible to assess the contribution of different substances to the fractions in question (Table 5). The results demonstrate that the prevalence of Cu2S and CuCO3 is characteristic of the samples after the first stage; however, minor amounts of CuSO4 and CuCl2 are also present there. The spectra of soil samples after the second stage with a high accuracy coincide with the Cu2S spectra. As for the soil sample after the third stage, it has a characteristically high content of CuCO3 (accounting for 50% of all Cu compounds) and Cu2S (30% of all Cu compounds) and low contents of CuSO4 (12% of all Cu compounds).
Figure 2c shows the Zn K-XANES spectra for the soil samples after different extraction stages versus the spectra of reference compounds, namely, ZnSO4, wurtzite (ZnS), and zincite (ZnO). The spectra of reference Zn-containing compounds with the known structures containing Zn–S (ZnS) and Zn–O (ZnO and ZnSO4) bonds display a considerable difference in the position of absorption edge, making it possible to distinguish between the types of Zn surroundings in Technosol samples.
The Zn K-XANES spectra of the soil sample after the third extraction stage are similar to the reference spectra, where Zn is coordinated by oxygen (ZnO and ZnSO4), with the absorption peak near 9670 eV. The XANES spectra of soil samples after the first two stages are close to ZnS, where the main specific absorption feature is near 9665 eV. However, it also contains higher energy variants, suggesting a possible presence of mixed Zn–S and Zn–O bonds in Technosol samples.
Similar to XANES, the Fourier-transforms (FT) of Zn K-edge EXAFS spectra in the samples of extracted fractions are rather distinct, suggesting different compounds of metal in these samples. The sample after the third fractionation stage is very similar to the analogous ZnSO4 spectrum, thereby confirming the results of analysis of XANES spectra. The absence of the peak corresponding to the second coordination sphere (Zn–Zn) suggests that ZnO is not the prevalent component in the sample. The FT EXAFS spectra of the soil samples after the first and second extraction stages show that the main peaks are shifted towards larger R values (Fig. 2d), suggesting that Zn–S bonds may be present in this sample; this matches the data of XANES analysis.
On the assumption that the Technosol samples after fractionation contain Zn–S and Zn–O phases, we made more precise quantitative assessments by fitting of the near-edge regions in the spectra of soil compounds using a linear combination of reference spectra. The results demonstrate that the main differences between fractions are associated with the ratio of ZnSO4 to ZnO contents. Zinc sulfate (ZnSO4) is the major component (65%) in the fraction after the first extraction stage, complying with the prevalence of Zn–O bond in the first sphere as suggested by the EXAFS data.
A high level of agreement between the linear combined spectra and experimental spectra of the sample of examined soil fractions demonstrates that ZnS is present in noticeable amounts in all samples. On the other hand, soil after the second and third stages contains 57 and 50% of Zn–S, respectively, in the form of mineral wurtzite (ZnS). According to the data on the upper horizon of heavily polluted soils in Palterton (United States), Zn was mainly fixed as sulfide–sphalerite (ZnS) [33].
Simulation of experimental Zn K-edge XANES spectra for Technosol after the second and third extraction stages demonstrated the presence of ZnO. As is observed, zincite (ZnO) is transformed in the soil it pollutes and is mainly present within Zn-containing trioctahedral structures [43].
The use of a linear combination of XANES and EXAFS spectra after each fractionation stage of Zn and Cu from heavily polluted Technosols has considerably improved the identification of specific metal species detectable according to the electron and molecular structures. EXAFS technique has shown the possibility of heterovalent isomorphic substitution of Al3+ ions in octahedral positions of clay minerals by Cu2+ and Zn2+ cations [28, 37].
CONCLUSIONS
The specific features of Cu and Zn transformation in the meadow-chernozem (Fluvisol) and technogenically transformed hydromorphic soils (Technosols) in the impact zone of the tailings pond are studied. The Cu and Zn analysis by sequential selective extractions shows that their distribution between the fractions is determined by the composition and properties of the examined soils, the level of pollution, and chemical properties of the metals. The highest amount of Cu concentrates in the residual fraction of Technosols and the fraction of Fe and Mn oxides. A specific feature in the speciation of metal compounds in the soils of the examined area is an increased content of pollutants in the fraction of crystalline Fe oxides as compared with the amorphous oxides and the prevalence of residual fraction, which suggests that the pollution is high and long-lasting.
The fractions retaining the metals in Technosols are identified by combining the analysis of XAFS spectra and XRD of the sample after each stage of BCR sequential extraction. As is shown, hydromorphic conditions and technogenic soil formation enhance the manifestation of HM siderophily. The use of a linear combination of XANES and EXAFS spectra after each stage of sequential Zn and Cu extraction in heavily polluted Technosols considerably improved the identification of metal speciation. A considerable difference in the position of absorption edge and the values of main specific features of the spectrum for the samples with Cu–S and Cu–O bonds makes it possible to reliably distinguish between these types of Cu surrounding in the samples of extracted fractions. XANES and EXAFS techniques has shown the possibility of heterovalent isomorphic substitution of the Al3+ in octahedral positions of clay minerals by Cu2+ and Zn2+ cations.
The methods using the synchrotron radiation are still infrequent in analyzing soils because soil has an intricate structure, is heterogeneous, and polydisperse. However, the ever-expanding potential of these methods suggests good reasons to expect the principally new knowledge about soils and the mechanisms underlying absorption of various chemical compounds by soils.
REFERENCES
A. L. Aleksandrovskii, “Role of time in the development and evolution of soils,” in National Soil Atlas (Moscow, 2011), pp. 66–67.
A. F. Vadyunina and Z. A. Korchagina, Practical Guide for Analysis of Physical Properties of Soils and Rocks (Agropromizdat, Moscow, 1986) [in Russian].
L. A. Vorob’eva, Theory and Practice of the Chemical Analysis of Soils (GEOS, Moscow, 2006) [in Russian].
Kh. M. Derkham, Candidate’s Dissertation in Biology (Moscow, 2009).
A. G. Zavarzina, N. N. Danchenko, V. V. Demin, Z. S. Artemyeva, and B. M. Kogut, “Humic substances: hypotheses and reality (a review),” Eurasian Soil Sci. 54, 1826–1854 (2021). https://doi.org/10.1134/S1064229321120164
D. V. Ladonin and M. M. Karpukhin, “Fractional composition of nickel, copper, zinc, and lead compounds in soils polluted by oxides and soluble metal salts,” Eurasian Soil Sci. 44, 874–885 (2011).
T. V. Pampura, D. L. Pinsky, V. G. Ostroumov, V. D. Gershevich, and V. N. Bashkin, “Experimental study of the buffer capacity of chernozem contaminated with copper and zinc,” Eurasian Soil Sci. 25, 27–38 (1993).
L. V. Perelomov and D. L. Pinskii, “Mn, Pb, and Zn compounds in gray forest soils of the Central Russian Upland,” Eurasian Soil Sci. 36, 610–618 (2003).
D. L. Pinsky, “Modern concepts about the absorption mechanisms of heavy metals by soils,” in Evolution, Functions, and Ecological Role of Soils as a Component of the Biosphere (Pushchino, 2020), pp. 55–64.
D. L. Pinskii, T. M. Minkina, S. S. Mandzhieva, Yu. A. Fedorov, T. V. Bauer, and D. G. Nevidomskaya, “Adsorption features of Cu(II), Pb(II), and Zn(II) by an ordinary chernozem from nitrate, chloride, acetate, and sulfate solutions,” Eurasian Soil Sci. 47, 10–17 (2014).
V. A. Kholodov, A. V. Kiryushin, N. V. Yaroslavtseva, and A. S. Frid, “Copper(II) binding by free and kaolinite-sorbed humic substances,” Eurasian Soil Sci. 47, 662–669 (2014).
M. Sh. Shaimukhametov, “Determination of adsorbed Ca and Mg in chernozems,” Pochvovedenie, No. 12, 105–111 (1993).
T. V. Bauer, D. L. Pinskii, T. M. Minkina, V. A. Shuvaeva, A. V. Soldatov, S. S. Mandzhieva, V. S. Tsitsuashvili, D. G. Nevidomskaya, and I. N. Semenkov, “Application of XAFS and XRD methods for describing the copper and zinc adsorption characteristics in hydromorphic soils,” Environ. Geochem. Health 44, 335–347 (2022). https://doi.org/10.1007/s10653-020-00773-2
M. Burachevskaya, T. Minkina, T. Bauer, S. Mandzhieva, C. Gülser, R. Kızılkaya, S. Sushkova, and V. Rajput, “Assessment of extraction methods for studying the fractional composition of Cu and Zn in uncontaminated and contaminated soils,” Eurasian J. Soil Sci. 9 (3), 231–241 (2020). https://doi.org/10.18393/ejss.734601
A. A. Chernyshov, A. A. Veligzhanin, and Y. V. Zubavichus, “Structural materials science end-station at the Kurchatov synchrotron radiation source: recent instrumentation upgrades and experimental results,” Nucl. Instrum. Methods Phys. Res., Sect. A 603 (1–2), 95–98 (2009). https://doi.org/10.1016/j.nima.2008.12.167
Z. C. Evans, H. V. Ryswyk, M. L. Huertos, and T. Srebotnjak, “Robust spatial analysis of sequestered metals in a Southern California Bioswale,” Sci. Total Environ. 650, 155–162 (2019). https://doi.org/10.1016/j.scitotenv.2018.08.441
E. Fernández-Ondoño, G. Bacchetta, A. M. Lallena, F. B. Navarro, I. Ortiz, and M. N. Jiménez, “Use of BCR sequential extraction procedures for soils and plant metal transfer predictions in contaminated mine tailings in Sardinia,” J. Geochem. Explor. 172, 133–141 (2017). https://doi.org/10.1016/j.gexplo.2016.09.013
M. Ghayoraneh and A. Qishlaqi, “Concentration, distribution and speciation of toxic metals in soils along a transect around a Zn/Pb smelter in the northwest of Iran,” J. Geochem. Explor. 180, 1–14 (2017). https://doi.org/10.1016/j.gexplo.2017.05.007
P. C. Gomes, M. P. F. Fontes, A. G. da Silva, E. S. Mendoca, and A. R. Netto, “Selectivity sequence and competitive adsorption of heavy metal by Brazilian soils,” Soil Sci. Soc. Am J. 65 (4), 1115–1121 (2001). https://doi.org/10.2136/sssaj2001.6541115x
L. Huang, Q. Jin, P. Tandon, A. Li, A. Shan, and J. Du, “High-resolution insight into the competitive adsorption of heavy metals on natural sediment by site energy distribution,” Chemosphere 197, 411–419 (2018). https://doi.org/10.1016/j.chemosphere.2018.01.056
T. A. Kirpichtchikova, A. Manceau, L. Spadini, F. Panfili, M. A. Marcus, and T. Jacquet, “Speciation and solubility of heavy metals in contaminated soil using X‑ray microfluorescence, EXAFS spectroscopy, chemical extraction, and thermodynamic modeling,” Geochim. Cosmochim. Acta 70 (9), 2163–2190 (2006). https://doi.org/10.1016/j.gca.2006.02.006
M. Leermakers, B. E. Mbacho, A. Husson, V. Lagneau, and M. Descostes, “An alternative sequential extraction scheme for the determination of trace elements in ferrihydrite rich sediments,” Talanta 199, 80–88 (2019). https://doi.org/10.1016/j.talanta.2019.02.053
A. Manceau, M. A. Marcus, and N. Tamura, “Quantitative speciation of heavy metals in soils and sediments by synchrotron X-ray techniques,” Rev. Miner. Geochem. 49 (1), 341–428 (2002). https://doi.org/10.2138/gsrmg.49.1.341
M. B. McBride, Environmental Chemistry of Soils (Oxford University Press, Oxford, 1994).
M. Mekapogu, J. Nadimikeri, P. K. Madri, and S. Devi, “A study on zinc speciation of Tungabhadra River sediments, Kurnool, south India: a tool in metal pollution monitoring,” Int. J. Sediment Res. 33 (4), 510–517 (2018). https://doi.org/10.1016/j.ijsrc.2017.11.001
P. W. Miller, D. C. Martens, and L. W. Zelazny, “Effect of sequence in extraction of trace metals from soils,” Soil Sci. Soc. Am. J. 50 (3), 598–601 (1986). https://doi.org/10.2136/sssaj1986.03615995005000030011x
T. Minkina, D. Nevidomskaya, T. Bauer, V. Shuvaeva, A. Soldatov, S. Mandzhieva, Y. Zubavichus, and A. Trigub, “Determining the speciation of Zn in soils around the sediment ponds of chemical plants by XRD and XAFS spectroscopy and sequential extraction.,” Sci. Total Environ. 634, 1165–1173 (2018). https://doi.org/10.1016/j.scitotenv.2018.04.118
T. Minkina, D. Nevidomskaya, M. Burachevskaya, T. Bauer, V. Shuvaeva, A. Soldatov, S. Mandzhieva, and Y. Zubavichus, “Possibilities of chemical fractionation and X-ray spectral analysis in estimating the speciation of Cu2+ with soil solid-phase components,” Appl. Geochem. 102, 55–63 (2019). https://doi.org/10.1016/j.apgeochem.2019.01.005
M. Misono, E. I. Ochiai, Y. Saito, and Y. Yoneda, “A new dual parameter scale for the strength of Lewis acids and bases with the evaluation of their softness,” J. Inorg. Nucl. Chem. 29 (11), 2685–2691 (1967). https://doi.org/10.1016/0022-1902(67)80006-X
F. Nannoni, G. Protano, and F. Riccobono, “Fractionation and geochemical mobility of heavy elements in soils of a mining area in northern Kosovo,” Geoderma 161 (1–2), 63–73 (2011). https://doi.org/10.1016/j.geoderma.2010.12.008
M. Pueyo, J. Mateu, A. Rigol, M. Vidal, J. F. López-Sánchez, and G. Rauret, “Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils,” Environ. Pollut. 152 (2), 330–341 (2008). https://doi.org/10.1016/j.envpol.2007.06.020
S. Salvador-Blanes, B. Minasny, and A. B. McBratney, “Modeling long-term in situ soil profile evolution: application to the genesis of soil profiles containing stone layers,” Eur. J. Soil Sci. 58 (6), 1535–1548 (2007). https://doi.org/10.1111/j.1365-2389.2007.00961.x
A. C. Scheinost, R. Kretzschmar, S. Pfister, and D. R. Roberts, “Combining selective sequential extractions, X-ray absorption spectroscopy, and principal component analysis for quantitative zinc speciation in soil,” Environ. Sci. Technol. 36 (23), 5021–5028 (2002). https://doi.org/10.1021/es025669f
G. Sere, Ch. Schwartz, S. Ouvrard, J.-Ch. Renat, F. Watteau, G. Villemin, and J. L. Morel, “Early pedogenic evolution of constructed Technosols,” J. Soils Sediments 10 (7), 1246–1254 (2010). https://doi.org/10.1007/s11368-010-0206-6
G. Y. Smolentsev and A. V. Soldatov, “Quantitative local structure refinement from XANES: multi-dimensional interpolation approach,” J. Synchrotron Radiat. 13 (1), 19–29 (2006). https://doi.org/10.1107/S0909049505038975
G. Sposito, The Chemistry of Soils (Oxford University Press, Oxford, 1989).
D. G. Strawn and L. L. Baker, “Speciation of Cu in a contaminated agricultural soil measured by XAFS, µ‑XAFS, and µ-XRF,” Environ. Sci. Technol. 42 (1), 37–42 (2008). https://doi.org/10.1021/es071605z
M. Sulkowski and A. V. Hirner, “Element fractionation by sequential extraction in a soil with high carbonate content,” Appl. Geochem. 21 (1), 16–28 (2006). https://doi.org/10.1016/j.apgeochem.2005.09.016
R. A. Sutherland, F. M. G. Tack, C. A. Tolosa, and M. G. Verloo, “Operationally defined metal fractions in road deposited sediment, Honolulu, Hawaii,” J. Eviron. Qual. 29 (5), 1431–1439 (2000). https://doi.org/10.2134/jeq2000.00472425002900050009x
B. Singh and M. Grafe, Synchrotron-Based Techniques in Soils and Sediments (Elsevier, Amsterdam, 2010).
P. G. C. Tessier and M. B. Campbell, “Sequential extraction procedure for the speciation of particulate trace metals,” Anal. Chem. 51 (7), 844–851 (1979). https://doi.org/10.1021/ac50043a017
A. Ure, P. H. Quevaullier, H. Muntau, and B. Griepink, “Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the CEC,” Int. J. Environ. Anal. Chem. 51 (1–4), 135–151 (1993). https://doi.org/10.1080/03067319308027619
A. Voegelin, G. Tokpa, O. Jacquat, K. Barmettler, and R. Kretzshmar, “Zinc fractionation in contaminated soils by sequential and single extractions: Influence of soil properties and zinc content,” J. Environ. Qual. 37 (3), 1190–1200 (2008). https://doi.org/10.2134/jeq2007.0326
A. Zwolak, M. Sarzyńska, E. Szpyrka, and K. Stawarczyk, “Sources of soil pollution by heavy metals and their accumulation in vegetables: a review,” Water, Air Soil Pollut. 230 (7), 1–10 (2019). https://doi.org/10.1007/s11270-019-4221-y
Funding
The work was supported by the Russia Science Foundation (grant no. 21-77-20089) at the Southern Federal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by G. Chirikova
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinskii, D.L., Minkina, T.M., Bauer, T.V. et al. Identification of Heavy Metal Compounds in Technogenically Transformed Soils Using Sequential Fractionation, XAFS Spectroscopy, and XRD Powder Diffraction. Eurasian Soil Sc. 55, 613–626 (2022). https://doi.org/10.1134/S1064229322050076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229322050076