Abstract
The experiments on mobilization of soil organic matter during soil washing with ultrafresh water against the background of salinity pulsing were designed and performed. Unpolluted soil and the soil artificially polluted with copper(II) were used in experiments, namely, clay loamy typical chernozem (Haplic Chernozem) of the Alekhin Central Chernozemic Nature Reserve (Kursk oblast, Russia; 51°34.207 N, 36°05.444 E) and sandy loamy soddy-podzolic soil (Albic Glossic Retisol (Loamic, Cutanic, Ochric)) from the Domodedovo district of Moscow oblast, Russia (55°17.683 N, 37°50.045 E). Soil samples were taken from the upper humus-accumulative (A1) horizon (5–15 cm). A drastic change in the composition of washing solution from fresh water to 0.1 M NaCl solution and back led to destruction of soil aggregates under the impact of osmotic pressure. Soddy-podzolic soil proved to be more resistant to destruction as compared with typical chernozem. Copper(II) was leached off from artificially contaminated samples of soddy-podzolic soil with the flow of dissolved organic matter, whereas copper leaching from typical chernozem was associated with the destruction of aggregates and release of intraaggregate organic matter. It is argued that copper (II) migration models should take into account the amount of dissolved organic matter for soddy-podzolic soil and the content of aromatic fragments in the organic matter for typical chernozem. A conceptual model of the Cu(II) leaching from contaminated soddy-podzolic soil and typical chernozem in the course of soil washing with fresh water against the background of salinity pulsing and the destruction of soil structure is constructed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The ever-increasing environmental pollution makes the research into the behavior of heavy metals (HMs), in particular, copper, in soil one of the most important challenges in the modern ecology. Copper is utilized in various fields of industry; it is an important trace element in manufacture of various products, and an active component in wood protectants and pesticides [17]. A wide use of copper determines frequent environmental pollution with this HM, soils included. Excess of copper ions in the soil can induce morphological, anatomical, and physiological alterations in plants and have a toxic effect on humans [2]. Sorption–desorption processes to a considerable degree determine the behavior of pollutants in soil, including copper ions [1, 16]. Currently, the immobilization of copper in humus horizons and its behavior in soils are relevant issues. Here, soil organic matter plays a special role. Note that the HM entering soil can bind to both soil organic matter and its particulate mineral component [6, 9, 24]. However, it is rather difficult to assess the individual contributions of its organic and mineral ingredients, which form an integrated humus–mineral complex.
Several papers demonstrate a higher affinity of the soil mineral part to copper ions [9, 15]. However, the organic components of colloids effectively block the mineral ones, preventing them from acting as binding centers, in the humus horizons with the content of organic matter of >1% [8]. In addition, soil organic matter is not static and can be mobilized when environmental conditions change [5]. The main factor for mobilization of organic matter to soil solution is soil washing with a large amount of ultrafresh water, for example, rains, heavy snowmelt, or runoff redistribution. A pulsed emission of readily soluble salts to soil solution can increase a damaging effect of fresh water [4, 6, 9–12, 14]. During mobilization, organic matter can be either dissolved or partially passes into washing water as fine particles. The latter are able to carry copper ions via colloidal transport. The dissolved organic matter, which has a high affinity for HMs as compared with the immobilized organic matter, increases the mobility of copper ions by forming soluble complexes with them [13, 19].
Note that the published information about the quantitative models of the above-described concurrent processes in soil is rather scanty. This is most likely associated with the difficulty in using any real soils in the saturated columns, where a constant flow of eluent exceeding the threshold values can cause degradation of water-stable aggregates and dissolution of organic substances. The most frequent variants studied are model columns to different degree approximating field/real/natural compounds, namely, the columns filled with glass or silicon beads, sand, or iron oxides [20, 21, 23, 25, 28]. Any estimates of the distribution of migrating HMs between two forms of soil organic matter are also absent. Correspondingly, it is difficult to assess the behavior of copper ions bound in the upper humus horizons.
The goal of the work was to study the mobilization of organic matter caused by extreme water–salt regimes in two soil types—typical chernozem and soddy-podzolic soil—as well as to assess the effect of this process on the behavior of copper ions during soil structure degradation. The copper mobility and quantitative leaching were assessed during organic matter mobilization both in dissolved state and within mobile colloids. This approach allows the copper behavior under the conditions most closely resembling the natural ones to be estimated and simulated.
EXPERIMENTAL
Characterization of soil samples. The samples of native soils—clay loamy typical chernozem and sandy loamy soddy-podzolic soil [3], or Haplic Chernozem (Loamic, Pachic) and Albic Glossic Retisol (Loamic, Cutanic, Ochric) according to the WRB classification [27]—were used in the work. Chernozem was sampled from the plots in the Streletskaya Step’ Nature Reserve area (Alekhin Central Chernozem Nature Reserve, Kursk oblast, Russia; 51°34.207 N, 36°05.444 E) and soddy-podzolic soil, in the Domodedovo district (Moscow oblast, Russia) in a forest stand aged at least 150 years (55°17.683 N, 37°50.045 E). Individual samples were taken from plots of approximately 5 m2 from the upper humus-accumulative horizon (A1) at a depth of 5–15 cm. Soil aggregates of the 3–5 mm fraction were used in experiments. The aggregates were obtained by sieving dry soil samples.
Column experiment on soil leaching with ultrafresh water on the background of changing salinity regime. The working hypothesis explaining the destruction of aggregates during changes in salinity regime relies on the assumption that the osmotic forces have no effect on structural units in the case of high salt concentration when the salinity of solution in the interaggregate space is equal to the salinity within aggregates. However, if the interaggregate solution is rapidly replaced with fresh water, the saline solution for a certain time is retained within the aggregates. Consequently, osmotic pressure is created in aggregates and leads to their destruction, release of intraaggregate organic matter, and its subsequent removal in dissolved and colloidal forms. Note that along with the osmotic effect, additional mechanisms can enhance the destruction of aggregates, including the dissolution of humus substances in the case when exchangeable calcium is replaced by sodium and the peptization of clay particles via an increase in the electrokinetic potential under saturation with exchangeable sodium on the background of a drastic decrease in the total salt concentration in solution. The destruction of aggregates was simulated in a column experiment with aggregates of soddy-podzolic soil and typical chernozem, which demonstrated the feasibility of aggregate “explosion” mechanism because of the change in osmotic pressure. In this experiment, we omitted a detailed description of the fresh water chemical composition and genesis. Chernozem was used as a potential target of the described destructive processes and soddy-podzolic soil, as a low-humus soil contrasting in the content of organic matter in order to expand the variants of conditions. In this experiment, a vertical glass column (d = 1.5 cm and h = 80 cm) was filled with soil aggregates and saturated with distilled water. The soil was slowly and gently added from the top. After filling up the column, it was stopped from the top with a plunger (a cylinder with a capillary for the eluent flow) to fix the precise height. The height of the column with soil was 70 cm and bulk density, 0.65 ± 0.05 g/cm3. After filling, the column was saturated with distilled water at a rate of 0.5 mL/min. The eluent was fed from the bottom upwards to remove the air from soil aggregates. After the saturation with eluent, the column was left for 12 h. Then the column was turned upside down so that the eluent would come from the top. This manipulation enhanced the passage of colloidal particles along the gravitational gradient.
To simulate the extreme leaching regime with salinity pulsing, observable in the presence of saline waters or rocks, the columns with soil were initially washed with distilled water followed by 0.1 M NaCl solution and again distilled water. The elution rate was 0.5 mL/min. This regime simulates the washing with ultrafresh water after snow melting followed by the salinization of soil solution during dry period caused by the raising of saline water and subsequent washing with rains. During the elution, the optical density of the solution coming out of the column was monitored with an UV detector at a wavelength of 254 nm. The washing solution was changed after the elution curve reached a plateau. The total volume of solutions used for the washing of soddy-podzolic soil was 5500 mL and for typical chernozem, 8500 mL. During the experiment, the solution running from the column was sampled at the key points (gradient increase in absorption, plateau, and replacement of washing solution) to assay for the content of dissolved (Cdis) and colloidal (Ccoll) organic matter.
The concentration of organic carbon in the dissolved and colloidal forms was determined in a Shimadzu TOC-4200 carbon analyzer. The carbon content in the Cdis organic matter was assayed after filtration of the eluent sample through a cellulose filter with a pore diameter of 0.45 µm. The carbon content in the Ccoll fraction was calculated as the difference between the total carbon content in the sample and Cdis.
To characterize the changes in the structure of organic matter during elution, the recorded data were used to calculate the parameter SUVA254 as the absorbance at 254 nm normalized to the content of organic carbon [26].
At the next stage, the mobilization of strongly bound HMs from soil resulting of the structural degradation was assessed. This was performed by the column experiments on the destruction of soil aggregates and monitoring of the release of interaggregate organic matter in the presence of HM pollution. These experiments were analogous to those described above but with artificially polluted soils. Copper (II), a classical HM for laboratory experiments [13], was selected for this purpose.
Preparation of model soil systems polluted with Cu(II). The experiment was performed as earlier described [22] in order to simulate the soil polluted with Cu(II) at a concentration exceeding the threshold limit value, amounting to 3 mg/kg [7]. The samples of 3–5 mm aggregates of soddy-podzolic soil and typical chernozem (200 g) were placed into a 1000-mL glass and saturated in a capillary manner with copper sulfate solution (6 g/L) by adding it dropwise onto the walls of the glass. After the soil was saturated, the volume of the added copper sulfate solution was adjusted to 200 mL. The soil was kept for 30 days with periodic stirring. On days 10 and 20 of incubation, the soil was moistened in a capillary manner by adding distilled water dropwise onto the glass walls, adjusting the total volume to 1 L, immediately decanting the water, and drying the sample.
After the incubation, the copper-polluted soil samples were again sieved (d = 3 mm) to prevent the column plugging by small soil aggregates. The column with a diameter of 1.5 cm was filled with the sieved aggregates. The height of the column with soddy-podzolic soil was 65 cm and soil weight, 86.5 g; in the case of typical chernozem, the height was 67 cm and soil weight, 69.5 g.
Total Cu(II) content was determined in soil samples before and after the column experiment as well as in the initial eluent and after its filtration through a cellulose filter (pore diameter, 0.45 µm). The HM content in soil was determined after treating the corresponding samples with a mixture of HCl and HF (3 : 1) in a Start D (Milestone, Italy) microwave digestion system. The Cu(II) content in the initial soil samples after the incubation was 3.47 and 5.68 mg Cu2+/kg for soddy-podzolic soil and typical chernozem, respectively. The Cu(II) concentrations in soil samples before and after washing at the column inlet, its middle part, and outlet were measured after pushing out the soil with compressed air. The soil was sampled from the top, middle part, and bottom of the resulting soil cylinder by quartering and analyzed for copper content. The copper content in solutions was determined by atomic absorption spectrometry in an Agilent 200 Series AA (Agilent Technologies, United States) spectrometer. These data were used to compute the content of Cu(II) associated with soil colloids and dissolved (presumably as chelates) carbon species.
Carbon and nitrogen contents were determined in the aggregates of soddy-podzolic soil and typical chernozem before and after elution. The samples were taken at the column inlet, its middle part, and outlet as described above and assayed for the content of total carbon and nitrogen by dry combustion in the oxygen flow in a Vario Macro CN automated analyzer [18].
RESULTS AND DISCUSSION
Assessment of the effect of salinity pulsing on the leaching of organic matter from soil columns. The changes in optical density of the washing solution and Cdis and Ccoll concentrations (estimated according to carbon concentration) depending on the eluent volume and composition, and sampling points are shown in Fig. 1. During the column experiments, samples were taken at the beginning of elution (25 mL), near 100 mL, when the optical density reached a plateau (1500 and 3000 mL for soddy-podzolic soil and typical chernozem, respectively), as well as immediately before and after addition (after 25 mL) of 0.1 M NaCl solution to the eluent. Then, one–two samples were taken when the elution curve reached the plateau in the elution with salt solution, one–two samples immediately (after 25 mL) after replacing the salt solution with distilled water, when the optical density decreased, and, if possible (as in the case of typical chernozem), again when the curve reached the plateau.
At the beginning of elution with distilled water, the optical density of solutions drastically increased and then rather soon dropped and reached the plateau. The observed effect in soddy-podzolic soil is mainly determined by the leaching of Cdis, the concentration of which in the maximum was 190 mg/L, while the Ccoll concentration increased insignificantly, reaching its maximum at 9 mg/L. In the case of chernozem, the contributions of carbon to the dissolved and colloidal matter at the beginning of washing were approximately equal: the maximum value for Cdis was 506 mg/L and for Ccoll, 408 mg/L. Addition of 0.1 M NaCl caused a decrease in optical densities of the both soil solutions relative to the plateau in the elution with distilled water, which is associated with a decrease in the leaching of organic matter caused by an increased ionic strength of solution. Note that the decrease in the flow of organic matter in soddy-podzolic soil is determined by a decrease in Cdis at a constant Ccoll value, which is explainable with an insignificant flow of carbon in a colloidal form. As for typical chernozem, this is explained by a concurrent decrease in the leaching of organic matter in both dissolved and colloidal states. After the salt solution is again replaced with distilled water, the optical density of the eluent drastically increased in both soddy-podzolic soil and typical chernozem. This fact is determined by an increase in leaching from soils of the intraaggregate organic matter in dissolved and colloidal forms, which is associated with the destruction of aggregates by osmotic pressure. When salt solution is replaced with distilled water, the interaggregate space contains distilled water, while 0.1 M salt solution is additionally present within aggregates. This creates a gradient of pressure directed from the aggregate outward, which induces destruction of aggregates. As is mentioned above, the dissolution of humus substances caused by the replacement of exchangeable calcium with sodium, along with the osmotic effect, may cause destruction of aggregates. The peptization of clay particles caused by an increase in electrokinetic potential under saturation with exchangeable sodium on the background of a drastic decrease in the total concentration of salts in solution may also contribute to this process.
The Ccoll of soddy-podzolic soil after a partial destruction of aggregates was almost twofold higher as compared with the Ccoll at the beginning of washing, amounting to 7.5 and 4 mg/L, respectively. The leaching of organic matter in a dissolved form was threefold lower as compared with the first maximum. As for typical chernozem, the destruction of aggregates caused a more considerable leaching of the dissolved organic matter as compared with the colloidal form. Thus, the dissolved intraaggregate organic matter is also mobilized along with the soil particles under the experimental conditions. Note that the structure of soddy-podzolic soil was not completely degraded under the extreme washing conditions. In the case of typical chernozem, the soil aggregates were almost completely destroyed with an elution volume of 8400 mL; the column was plugged; and the experiment stopped. Presumably, a smaller degree of destruction of the soddy-podzolic soil aggregates as compared with typical chernozem is explainable by that soddy-podzolic was formed under the percolative water regime in the boreal belt, which makes it more stable to washing as compared with typical chernozem, a steppe soil.
To assess the leaching of organic matter caused by salinity pulsing, the contents of organic carbon and nitrogen in the washing column were measured before and after the experiment (Table 1).
The mean carbon content over the column after washing was 1.95% for soddy-podzolic soil and 4.67% for chernozem. The mean nitrogen content was 0.21 and 0.46%. According to t-test, the differences between the contents of organic carbon and nitrogen before and after washing the column (Corg for soddy-podzolic soil, 3.26 and 1.95% and for typical chernozem, 6.43 and 4.97% and N for soddy-podzolic soil, 0.21 and 0.32% and for typical chernozem, 0.46 and 0.66%) are statistically significant (α = 0.05). Thus, the losses of soil carbon in the experiment were considerable, amounting to 1.3 and 1.8% for soddy-podzolic soil and typical chernozem, respectively. The corresponding losses of organic nitrogen were rather insignificant, namely, 0.1 and 0.2%, respectively. The C/N ratio was almost constant in soddy-podzolic soil, suggesting that the qualitative composition of its organic matter did not change after washing under the used conditions. A minor change in the C/N ratio in typical chernozem may suggest a qualitative difference in the structure of organic carbon removed from the soil. Correspondingly, the composition of the Cdis organic matter leached from typical chernozem was estimated according to the dependence of SUVA254 on the volume used for elution (Fig. 2).
The pattern of the change in SUVA254 value on the elution volume suggests that highly aromatic organic matter is mobilized when column is washed with distilled water, while the organic matter with a decreased aromaticity is mobilized after soil aggregates are destroyed. An increased aromaticity is usually associated with more humified organic matter. Thus, a decrease in the SUVA254 value after the destruction of aggregates indicates that the organic matter in aggregate interiors is less transformed.
Elution of the soil columns polluted with copper. The Cu(II) pollution of the examined soil samples did not significantly change the elution curves as compared with the unpolluted samples. In general, the leaching profile of carbon compounds for the soil samples polluted with Cu(II) is close to that for their unpolluted analogs (Fig. 3).
Dependences of the concentration of organic carbon in dissolved (Cdis) and colloidal (Ccoll) forms on the volume of eluent and its replacement for (a) soddy-podzolic soil (0–1500 mL of distilled water followed by 1500–1800 mL of 0.1 M NaCl solution and finally distilled water) and (b) typical chernozem (0–3150 mL of distilled water followed by 3200–4000 mL 0.1 M NaCl solution and finally distilled water).
In the case of elution with distilled water, the Cdis concentration in soddy-podzolic soil at the initial stage changed from 380 to 11 mg/L when reaching the plateau, while the Ccoll concentration at the beginning of experiment reached 51 mg/L and then dropped almost to zero. In soddy-podzolic soil, the leaching of organic matter (both dissolved and colloidal) was more intensive as compared with the unpolluted sample. Presumably, the Cu(II) sorbed in soil enters solution and enhances mobilization of organic carbon.
After distilled water was replaced with 0.1 M NaCl, Cdis and Ccoll concentrations insignificantly decreased most likely because of rather small carbon flux (both colloidal and dissolved сarbon). The further change in washing solution for distilled water caused partial destruction of aggregates in soddy-podzolic soil, which appeared as a sharp increase in leaching of colloidal carbon. A 12-fold increase in the Ccoll concentration as compared with the initial stage of elution was observed, whereas the concentration of Cdis increased insignificantly. Any drastic increase in the leaching of colloidal carbon after replacing the salt solution with distilled water was unobservable in the unpolluted soddy-podzolic soil. As for the Cu(II)-polluted soddy-podzolic soil, the leaching of colloidal carbon after destruction of aggregates was more intensive as compared with the dissolved carbon, suggesting that the structure of polluted soils is less stable under the used conditions. Thus, the pollution of soddy-podzolic soil with Cu(II) ions decreases the resistance of its structure to the damaging impact of washing with fresh water on the background of the changes in salinity.
A comparison of the distribution of unpolluted (Fig. 1d) and Cu(II)-polluted (Fig. 3b) organic matter flows for typical chernozem shows that the dependences are rather similar. Note that the soil structure of Cu(II)-polluted typical chernozem was destroyed considerably more rapidly, while the destruction of unpolluted soil structure took a longer time.
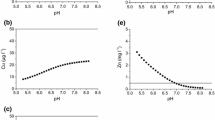
Two mobile compounds of HM—(1) bound to soil colloidal matter (Cucoll) and migrating (Cufree) with soil particles and (2) freely migrating, moving in a dissolved form as free ions or bound to organic matter—were studied to find out the form of copper leaching from soil samples when the structure gets degraded. Figure 4 shows the leaching curves for copper in a free form and within colloidal particles for soddy-podzolic soil and typical chernozem.
Dependences of the concentration of copper in free organic matter (Cufree) and organic matter bound to colloidal particles (Cucoll) on the volume of eluent and its replacement for (a) soddy-podzolic soil (0–1500 mL of distilled water followed by 1500–1800 mL of 0.1 M NaCl solution and finally distilled water) and (b) typical chernozem (0–3150 mL of distilled water followed by 3200–4000 mL 0.1 M NaCl solution and finally distilled water).
As is evident from the shown data, the main part of Cu(II) in soddy-podzolic soil is leached at the beginning of the experiment. As for typical chernozem, the maximum of Cu(II) leaching is observed after destruction of soil aggregates caused by NaCl. The concentration of leached HM is higher in the case of chernozem. In both variants, a large part of Cu(II) is leached in a dissolved form, presumably, within the mobile complexes with organic matter since the leaching peaks match the peaks of removal of dissolved organic matter (Fig. 3). However, quasi-equilibrium is established in soddy-podzolic soil with rather insignificant Cu(II) leaching and washing volume of 500–1500 mL and the elution curve reaches a plateau. Although the leaching of dissolved organic matter in this situation is governing the carbon content, Cu(II) is mainly removed within colloidal particles. As for typical chernozem, the maximums of leaching of both Cu(II) variants are observed after the structure is destroyed; moreover, initially, the removal of Cu(II) is insignificant or absent at all.
The observed differences and specific features of the HM leaching from soddy-podzolic soil and typical chernozem (assuming that the added sodium chloride causes osmotic destruction of aggregates and subsequent release of intraaggregate organic matter) are explainable using a conceptual model of the Cu(II) leaching from polluted soddy-podzolic soil and typical chernozem.
(1) In the case of typical chernozem with a sufficiently high pollution level (when the equilibrium is reached), Cu(II) prevalently binds to intraaggregate organic matter. As for soddy-podzolic soil, part of Cu(II) also binds to intraaggregate organic matter but its major part is associated with readily leached (water-soluble) organic carbon.
(2) Fresh water almost does not leach Cu(II) from typical chernozem. On the contrary, a major part of this HM is leached from soddy-podzolic soil by fresh water, presumably, as the ingredient of chelates with dissolved organic matter.
(3) A small pool of the Cu(II) associated with colloidal organic matter is constantly leached from soddy-podzolic soil during a long-term washing; however, this is not the case for typical chernozem.
(4) The dissolved Cu(II) most likely associated with mobile organic matter is the prevalent HM form leached from soil.
(5) The main Cu(II) amount leached from typical chernozem is associated with degradation of soil structure and release of intraaggregate dissolved organic matter.
(6) The structure of soddy-podzolic soil is more resistant to the destruction caused by the osmotic pressure formed in aggregates as compared with typical chernozem.
Correlation analysis of the amount of organic matter leached from the examined soil samples and Cu(II) compounds (Table 2) demonstrates a tight correlation of the amount of leached HM (in free and colloidal forms) with the content of dissolved organic matter in eluent in the case of soddy-podzolic soil (r = 0.94 and 0.63, respectively).
However, any correlation between the colloidal and dissolved organic matter in eluent was unobservable. Presumably, this is associated with competitive interactions at the moment when the Cu(II) mobilized by the dissolved organic matter is leached concurrently with colloidal particles.
On the other hand, this interaction pattern was unobservable for typical chernozem. However, a tight correlation (r = 0.85) between the leaching patterns of Cucoll and Cufree was observed, presumably resulting from the dependence of HM leaching on soil structure degradation when all Cu(II) compounds are leached. The correlation (r = 0.88) between the leaching of organic matter in a dissolved form and within colloids confirms this assumption.
Of interest is the absence of correlation between the SUVA254 value and Cu(II) behavior. This fact may be associated with an equilibrium pattern of pollution: soil samples were treated with Cu(II) solution at a concentration threefold higher than the threshold limit value followed by 30-day incubation with moistening to field water capacity once every 7 days. Under these conditions, the Cu(II) behavior will be primarily determined rather by the mode of its existence (free dissolved or colloidal) than by the structure of organic matter, as well as the corresponding Cu(II) binding constants. Note that the constant of Cu(II) binding to dissolved organic matter is by two orders of magnitude higher as compared with colloidal organic matter [13].
Assessment of the Cu(II) amount leached from soil column demonstrated that the mean HM content in the column after washing was 3.25 mg/kg for soddy-podzolic soil and 3.23 mg/kg for typical chernozem (Table 3); the corresponding values before washing were 3.47 and 5.68 mg/kg. Statistical analysis (t-test) demonstrated that the Cu(II) concentrations before and after washing differed in a statistically significant manner (α = 0.05) for both soils (soddy-podzolic soil and typical chernozem).
The performed experiment has shown that only 6% of the applied HM was leached from soddy-podzolic soil versus 43% leached from typical chernozem. Presumably, this large amount of Cu(II) leached from the column with typical chernozem is explainable by intensive degradation of its structure. In general, the Cu(II) distribution along the column height for both soddy-podzolic soil and typical chernozem suggests a low mobility of the metal in the examined soils. Presumably, this is associated with the Cu(II) redeposition in the lower layers of soil samples during the migration along the columns. As is evident from Table 3, the Cu(II) content at the column outlet was close to its concentration in the initial soil samples.
CONCLUSIONS
The change in the composition of soil solution during intensive washing with fresh water followed by its saturation with salt and subsequent switch back to fresh water destroys soil aggregates in soddy-podzolic soil and typical chernozem. This is explainable with the creation of a high osmotic pressure in soil aggregates interiors directed from the center of aggregates to the interaggregate pores (cracks). In addition to the osmotic effect, the dissolution of humus substances resulting from the replacement of exchangeable calcium by sodium may enhance the destruction of soil aggregates as well as the peptization of clay particles because of an increase in the electrokinetic potential under saturation with exchangeable sodium on the background of a drastic decrease in the total salt content of the solution.
The aggregates of soddy-podzolic soil are more resistant to such destruction as compared to the aggregates of typical chernozem. Most likely, this is associated with the percolative regime during formation of soddy-podzolic soil when the most water-stable structures are selected.
Under equilibrium conditions, Cu(II) in typical chernozem is mainly bound to intraaggregate organic matter, which is untypical of soddy-podzolic soil.
Washing of the Cu(II)-polluted soddy-podzolic soil with fresh water enhances the active leaching of HM, whereas Cu(II) is almost not leached from typical chernozem under these conditions.
The leaching of HM in the typical chernozem polluted with copper is associated with the its structure degradation and the release of intraaggregate organic matter.
As for the soddy-podzolic soil polluted with Cu(II), the leaching of HM is directly associated with the flow of dissolved organic matter. After the main part of dissolved organic matter is leached from soddy-podzolic soil by fresh water, this fresh water carries out Cu(II) mainly within colloidal particles. However, Cu(II) is not leached from typical chernozem under these conditions.
In soddy-podzolic soil, immobilized HM is competitively bound by dissolved organic matter. This fact is confirmed by a tight correlation of Cdis with free copper compounds, Cudis (r = 0.94), and the absence of correlation between the flow of organic matter in a colloidal form and the copper content in mobile colloidal particles. In the case of typical chernozem, the flows of free Cu(II) and the Cu(II) bound to mobile particles are independent of the quantitative characteristics of the flows of organic carbon both colloidal and dissolved.
REFERENCES
M. V. Dabakhov, E. V. Dabakhova, and V. I. Titova, Heavy Metals: Ecotoxicology and Standardization (Volgo-Vyatka Academy of Public Administration, Nizhny Novgorod, 2005) [in Russian].
A. Kabata-Pendias and H. Pendias, Trace Elements in Soils and Plants (CRC Press, Boca Raton, FL, 1984; Mir, Moscow, 1989).
Classification and Diagnostics of Soils of the USSR (Kolos, Moscow, 1977) [in Russian].
S. P. Kravkov, Biochemistry and Agrochemistry of Soil Processes (Nauka, Leningrad, 1978) [in Russian].
A. Calderbank, “The occurrence and significance of bound pesticide residues in soil,” in Reviews of Environmental Contamination and Toxicology, Ed. by G. Ware (Springer-Verlag, New York, 1989; Mir, Moscow, 1993), pp. 71–103.
V. G. Petrov, M. A. Shumilova, M. V. Lopatina, and V. A. Aleksandrov, “Sorption of copper (2+) ions in soils,” Vestn. Udmurt. Gos. Univ., Ser. Fiz., Khim., No. 2, 74–77 (2012).
Maximum Permissible Concentrations (MPCs) of Chemical Substances in Soil (Federal Hygienic and Epidemiological Center of Rospotrebnadzor, Moscow, 2006) [in Russian].
Reviews of Environmental Contamination and Toxicology, Ed. by G. Ware (Springer-Verlag, New York, 1989; Mir, Moscow, 1993).
O. B. Rogova and Yu. N. Vodyanitskii, “Zinc and copper sorption in soils affected by Cherepovetsk metallurgical complex,” Byull. Pochv. Inst. im. V.V. Dokuchaeva, No. 65, 65–74 (2010).
D. S. Orlov, L. K. Sadovnikova, and N. I. Sukhanova, Soil Chemistry (Vysshaya Shkola, Moscow, 2005) [in Russian].
G. N. Fedotov and G. V. Dobrovol’skii, “Possible ways of nanostructure development in soil gels,” Eurasian Soil Sci. 45, 811–822 (2012).
N. B. Khitrov, Overmoistening of Chernozems in Automorphic Steppe Agrolandscapes as a Result of Human Economic Activities (Orenburg, 2003), pp. 551–554.
V. A. Kholodov, A. V. Kiryushin, N. V. Yaroslavtseva, and A. S. Frid, “Copper(II) binding by free and kaolinite-sorbed humic substances,” Eurasian Soil Sci. 47, 662–669 (2014).
E. V. Shein and B. A. Devin, “Current problems in the study of colloidal transport in soil,” Eurasian Soil Sci. 40, 399–408 (2007).
M. Arias, M. T. Barral, and J. C. Mejuto, “Enhancement of copper and cadmium adsorption on kaolin by the presence of humic acids,” Chemosphere 48, 1081–1088 (2002). https://doi.org/10.1016/S0045-6535(02)00169-8
G. Brunetto, G. W. Bastos de Melo, R. Terzano, D. Del Buono, S. Astolfi, N. Tomasi, Y. Pii, T. Mimmo, and S. Cesco, “Copper accumulation in vineyard soils: rhizosphere processes and agronomic practices to limit its toxicity,” Chemosphere 162, 293–307 (2016). https://doi.org/10.1016/j.chemosphere.2016.07.104
“Canadian soil quality guidelines for the protection of environmental and human health: copper,” in Canadian Environmental Quality Guidelines (Canadian Council of Ministers of the Environment, Winnipeg, MB, 1999).
ISO 10694:1995: Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis) (International Organization for Standardization, Geneva, 1995).
C. P. Jordao, C. Reis, C. R. Bellato, and J. L. Pereira, “Adsorption of Cu2+ ions on humic acids,” REM, Rev. Esc. Minas 54 (2), 109–114 (2001). https://doi.org/10.1590/S0370-44672001000200006
K. Hanna, L. Lassabatere, and B. Bechet, “Zinc and lead transfer in a contaminated roadside soil: experimental study and modeling,” J. Hazard Mater. 161 (2–3), 499–505 (2009). https://doi.org/10.1016/j.jhazmat.2008.04.124
K. Mitchell, E. Moreno-Jimenez, R. Jones, L. Zheng, L. Trakal, R. Hough, and L. Beesley, “Mobility of arsenic, chromium and copper arising from soil application of stabilized aggregates made from contaminated wood ash,” J. Hazard. Mater. 393, 122479 (2020). https://doi.org/10.1016/j.jhazmat.2020.122479
G. V. Motuzova, T. M. Minkina, E. A. Karpova, N. U. Barsova, and S. S. Mandzhieva, “Soil contamination with heavy metals as a potential and real risk to the environment,” J. Geochem. Explor. 144, 241–246 (2014). https://doi.org/10.1016/j.gexplo.2014.01.026
K. Pawluk, J. Fronczyk, and K. Garbulewski, “Experimental development of contaminants removal from multicomponent solutions using ZVI, zeolite and modified construction aggregate—batch and column tests,” Desalin. Water Treat. 144, 89–98 (2019). https://doi.org/10.5004/dwt.2019.23544
C. Qu, W. Chen, X. Hu, P. Cai, C. Chen, X.-Y. Yu, and Q. Huang, “Heavy metal behavior at mineral-organointerfaces: mechanisms, modeling and influence factors,” Environ Int. 131, 104995 (2019). https://doi.org/10.1016/j.envint.2019.104995
L. Sun, Y. Xue, C. Peng, C. Xu, and J. Shi, “Influence of sulfur fertilization on CuO nanoparticles migration and transformation in soil pore water from the rice (Oryza sativa L.) rhizosphere,” Environ. Pollut. 257, 113608 (2020). https://doi.org/10.1016/j.envpol.2019.113608
J. L. Weishaar, G. R. Aiken, B. A. Bergamaschi, M. S. Fram, R. Fujii, and K. Mopper, “Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon,” Environ. Sci. Technol. 37 (20), 4702–4708 (2003). https://doi.org/10.1021/es030360x
IUSS Working Group WRB, World Reference Base for Soil Resources 2014, Update 2015, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, World Soil Resources Reports No. 106 (UN Food and Agriculture Organization, Rome, 2015).
H. Wu, L. Hu, and G. Zhang, “Effects of electro-osmosis on the physical and chemical properties of bentonite,” J. Mater. Civil Eng. 28 (8), 06016010 (2016). https://doi.org/10.1061/(ASCE)MT.1943-5533.0001572
ACKNOWLEDGMENTS
The work was performed using the equipment of the joint access center “Functions and Properties of Soils and Soil Cover” with the Dokuchaev Soil Science Institute, Russian Academy of Sciences.
Funding
Sampling and characterization of soils were supported by the Russian Science Foundation (project no. 19-16-00053) and column experiments were preformed under budget projects of the Gubkin National University of Oil and Gas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state no conflicts of competing financial interest or personal relations that could affect the work described in this paper.
Additional information
Translated by G. Chirikova
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grechishcheva, N.Y., Yaroslavtsev, N.V., Kotelnikova, A.D. et al. Mobilization of Soil Organic Matter by Ultrafresh Water: Modeling and Assessment of the Impact on the Mobility of Heavy Metals. Eurasian Soil Sc. 54, 843–851 (2021). https://doi.org/10.1134/S1064229321060053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229321060053