Abstract
The spherical green alga Volvox consists of several hundred or thousand of somatic cells that undergo terminal differentiation, senescence and death, and a small number of gonidia (asexual reproductive cells) that give rise to the next generation. In the first part of this paper, the ontogenetic diversity of the genus Volvox is briefly considered, as well as the mechanisms of differentiation into the two types of cells mentioned above, which have been thoroughly studied during recent years in Volvox carteri. Then, a detailed critical analysis of the literature and some of my own data on senescence and cell death (mainly in V. carteri and, to a lesser extent, in V. aureus) was carried out, and it was noted that this aspect of Volvox developmental biology has not been sufficiently studied. Some perspectives of further research of the processes of cell death and senescence in representatives of the genus Volvox in a comparative aspect are indicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The processes of senescence and cell death have been studied in recent years using various model organisms and systems: multicellular animals (Goldsmith, 2015; Brusentsev et al., 2017; Davaapil et al., 2017; Skulachev, M.V. and Skulachev, V.P., 2017; Zhu et al., 2019; etc.), higher plants (van Doorn and Woltering, 2004; Rogers, 2015; Locato and De Gara, 2018; Woo et al., 2019; Doronina et al., 2020; etc.), unicells, including protists (Gordeeva et al., 2004; Franklin et al., 2006; Deponte, 2008; Kasuba et al., 2015; Bidle, 2016; Durand et al., 2016). Among the model organisms (systems) for the analysis of this problem, one of the most primitive multicellular (colonial) organisms, the volvocine algae, could occupy a rather important place, and this was traditionally noted in the literature (see, for example: Weismann, 1893, 1904; Golstein et al., 2003; Bhatia-Dey et al., 2016). Nevertheless, the first experimental studies of the senescence in the alga Volvox (Chlorophyta, Volvocales, Volvocaceae) were published about 40 years ago (Hagen and Kochert, 1980; Pommerville and Kochert, 1981, 1982), subsequent works on this topic appeared only sporadically and there were no special overview publications on cell death and senescence in this typical representative of the phytoplankton of lentic freshwaters. This article first summarizes some of the current data on the developmental biology of Volvox (which are important for understanding the main topic of the review), and then presents a discussion of the literature data and some of my own data on cell death and senescence in Volvox. It will expand our understanding of the features of these processes in lower plants.
Speaking about senescence and cell death in Volvox, as a rule, the researchers mean the age-related changes in somatic cells during the development of the model species V. carteri. In this article, for the first time in the literature on senescing in Volvox, an attempt is made to apply a comparative approach and draw data on the “non-model” species V. aureus. Finally, I would also like to draw attention to the peculiarities of the death of gonidia (potentially immortal asexual reproductive cells) when the resources in aging cultures of different Volvox species are limited.
BASIC DATA ON DEVELOPMENT AND CELL DIFFERENTIATION IN VOLVOX
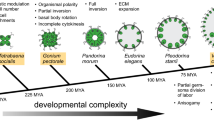
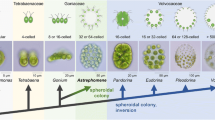
The genus Volvox includes more than 20 species (Herron and Nedelcu, 2015; Nozaki et al., 2015, 2019, 2020) and these green flagellate algae provide an opportunity to analyze individual development in a system consisting of only two cell types: several hundreds or thousands of small somatic cells and a small number (usually 8–16) of large reproductive cells. Despite the relative simplicity of the organization of Volvox (Fig. 1), researchers are faced here with a number of interesting phenomena and processes: the formation of cellular lines, the growth and division of reproductive cells, reorganizations of ontogeny in the evolution of related species, morphogenesis, sexual pheromones, etc. Thus, Volvox has become a rather popular model organism of developmental biology—a number of review articles in recent years are devoted to the above-mentioned aspects of ontogeny of this alga (Herron, 2016; Matt and Umen, 2016; Desnitskiy, 2018, 2019; Desnitskiy et al., 2018; Umen, 2020; etc.).
The cycle of asexual development of Volvox, which is studied under the conditions of clonal axenic cultures (containing only one species of microorganism), includes the growth of gonidia (asexual reproductive cells), the period of their division (a series of 9–15 synchronous divisions in various species), inversion (turning inside out) of young spheroids, their growth inside the parental individual, release from the parental spheroid, after which the gonidia of the next generation undergo growth and cleavage. On the other hand, the remaining spherical hulk, consisting only of the parental soma, grows old and dies. Traditionally, two main types of asexual development and reproduction have been distinguished in Volvox (Starr, 1970; Kochert, 1975; Desnitski, 1992, 1995; Desnitskiy, 2016). We can also talk about two reproductive strategies. In several species (V. africanus, V. carteri f. kawasakiensis, V. carteri f. nagariensis, V. carteri f. weismannia, V. gigas, V. spermatosphaera), gonidia as a result of a prolonged period of light-dependent growth reach large size before the onset of cleavage and exceed somatic cells in diameter by at least 6–8 times. During rapid successive divisions (the entire cleavage period takes no more than 10–12 hours), which can occur in the dark, there is no cellular growth. On the contrary, in other species (for example, V. aureus, V. ferrisii, V. globator, V. rousseletii), mature gonidia are relatively small and exceed somatic cells in the diameter by no more than 3–4 times, since the period of gonidial growth is short and the embryonic cells grow during long intervals between cleavage divisions that require light. Consequently, in this case, the cleavage period is extended by at least two or three days. The divisions start in the morning of the first day, are temporarily blocked at night, resume in the morning of the next day, stop again at night, etc. (Desnitskiy, 2017). Phylogenetic analysis (Herron et al., 2010) shows that this type of asexual development in Volvox (with slow and light-dependent cleavage) is evolutionarily advanced.
The cycles of sexual ontogenesis in Volvox include the development and differentiation of spheroids with male or female reproductive cells (instead of asexual gonidia). The only diploid stage in the Volvox life cycle is the zygote (zygospore), which germinates after a dormant period (Starr, 1975). Most representatives of the genus Volvox have species-specific sexual pheromones (Starr, 1970; Kirk, 1998; Hallmann, 2011; Coleman, 2012). In particular, under the influence of such a pheromone produced by a spontaneously formed male or somatic cells of an asexual individual under stressful conditions (for example, heat shock or mechanical injury), the asexual gonidia of V. carteri f. nagariensis undergo a modified cleavage pattern and form sexual individuals (with eggs in the female clone or with androgonidia in the male clone) in the next generation.
Returning to a brief description of the asexual developmental cycle, let us turn to the problem of differentiation into types of reproductive and somatic cells. First of all, we note that the presence of somatic cells in the Volvox spheroid is an undoubted feature of a multicellular organism. At the same time, a distinctive feature and advantage of this organism (in comparison, for example, with multicellular animals or higher plants) is that the soma in Volvox is represented by only one type of cells. According to phylogenetic analysis (Herron and Michod, 2008), the soma arose three times during the evolution of algae of the order Volvocales, and twice within the polyphyletic genus Volvox. The main functions of Volvox biflagellated somatic cells are the provision of motility of the organism (including phototaxis) and the synthesis of the glycoproteinaceous extracellular matrix that maintains the organization of the spheroid with both cellular types (Matt and Umen, 2018). There was an idea (Koufopanou and Bell, 1993; Koufopanou, 1994; Hoops et al., 2006) about the possibility of transferring nutrients from young somatic cells (synthesized by them or even “picked up” from the environment) to gonidia and cleaving embryos, though this is still not conclusively proven.
The embryos of V. africanus, V. carteri f. kawasakiensis, V. carteri f. nagariensis, V. carteri f. weismannia, V. obversus and V. reticuliferus are characterized by asymmetric (unequal) divisions and differentiation of lines of presumptive reproductive and somatic cells at relatively early stages of development: at 16-celled, 32‑celled, or at slightly later stages of cleavage (Starr, 1969; Karn et al., 1974; Nozaki, 1988; Herron et al., 2010; Desnitskiy, 2016). In embryos of other Volvox species, there are no asymmetric divisions, and gonidia become morphologically distinguishable from somatic cells only after cleavage is completed or even after inversion of the young spheroid.
We will now proceed to a brief presentation of data on the cellular and molecular genetic mechanisms of differentiation into soma and gonidia in V. carteri f. nagariensis, the only representative of the genus studied in detail in this respect (Tam and Kirk, 1991; Kirk, 1998, 2001, 2005; Pappas and Miller, 2009; Matt and Umen, 2016; etc.). When an embryo of this species and forma of Volvox reaches the 32-celled stage of cleavage (after five symmetric divisions), the 16 anterior cells divide asymmetrically into large and small cells, the lines of presumptive gonidial and somatic cells of the next generation respectively. By contrast, the 16 cells in the posterior part of the embryo undergo only symmetric divisions and thus contribute only to the formation of the small somatic cell line. The asymmetric divisions in anterior embryonic cells occur under the control of the glsA and Hsp70A genes, which encode chaperone proteins (glsA and Hsp70A, respectively) interacting with the mitotic spindle and displacing the plane of cell division (Kirk et al., 1999; Cheng et al., 2005; Pappas and Miller, 2009). Interestingly, there are several studies that have shown the involvement of the glsA gene in male gametogenesis (Mori et al., 2003; Igawa et al., 2009) in Liliales or in other morphogenetic processes in higher plants (Guzman-Lopez et al., 2016).
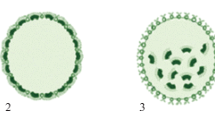
During the development of V. carteri f. nagariensis embryo, after the asymmetric cleavage, the lag gene products repress the activity of somatic genes in large cells, while in small cells the regA gene products repress the activity of gonidial genes (Kirk, 2001; Matt and Umen, 2016). Consequently, the large and small cells of the embryo differentiate into large reproductive cells (asexual gonidia) and small somatic cells, respectively. Previous experimental studies (Pall, 1975; Kirk et al., 1993) showed that all cells of the embryo reaching 8 μm in diameter or more at the end of the series of divisions become gonidia (regardless of their position in the spheroid), and all cells less than 8 μm in diameter differentiate as somatic cells. Note that during normal development in V. carteri f. nagariensis, the prospective gonidia at the end of a series of divisions are about 13 μm in diameter, and young somatic cells are about 2 μm. Thus, an important factor determining the direction of cellular differentiation in the young spheroid of this Volvox species is cell size. However, the reason for the direct connection between the size of the embryonic cell and the determination of its fate remains mysterious. Formal scheme of the process of differentiation into gonidia and somatic cells in V. carteri f. nagariensis is shown in Fig. 2.
Of all the genes in V. carteri f. nagariensis mentioned above, the regA gene, which controls somatic differentiation, prevents chloroplast biogenesis, and encodes a transcription repressor located in the nucleus, has been studied in most detail (Tam et al., 1991; Choi et al., 1996; Kirk et al., 1999; Stark et al., 2001; Duncan et al., 2007; Grochau-Wright et al., 2017; etc.). In the light of these data, it becomes clear why the content of chloroplast DNA in the gonidium before the beginning of the cleavage period exceeds the content of chloroplast DNA in the somatic cell by about 120 times (Kochert, 1975). It is interesting to note that in the regA– mutant lines, morphologically normal young asexual spheroids with both cellular types are initially formed. However, later somatic cells increase in size, transdifferentiate into gonidia and undergo a series of cleavage divisions. This group of mutations in V. carteri f. nagariensis was first described about 50 years ago and was then called the “somatic regenerator” (Starr, 1970).
Interestingly, Hanschen et al. (2014) recently identified the regA gene in several other representatives of the polyphyletic genus Volvox, including V. ferrisii, a species with a small size of mature gonidia and no asymmetric division. The last common ancestor of V. carteri f. nagariensis and V. ferrisii did not have somatic cells (Herron and Michod, 2008) and, thus, soma arose independently in two evolutionary lines. The regA gene, crucial for the somatic differentiation in V. carteri f. nagariensis, has a homologue in the unicellular related alga Chlamydomonas reinhardtii from the order Volvocales. In C. reinhardtii, this homologous gene is expressed under stressful conditions, when cell growth and reproduction are suppressed (Nedelcu and Michod, 2006; Nedelcu, 2009a; König and Nedelcu, 2020). On the other hand, the lag gene, which is important for the differentiation of gonidia in V. carteri f. nagariensis, has not yet been the subject of a thorough study (in contrast to the regA, glsA, and Hsp70A genes), and it was relatively recently noted (Matt, Umen, 2016) that the specific mechanism of action of this gene in differentiation into two cell types is still unclear.
SENESCENCE AND CELL DEATH IN VOLVOX
Volvox somatic cells are terminally differentiated post-mitotic cells undergoing senescence and death (Starr, 1970; Kochert, 1975; Kirk, 1998; Matt and Umen, 2016; etc.). They are unable to divide after the completion of the processes of cleavage and inversion of the embryo. The description of somatic cell division in V. aureus available in the literature (Soyer, 1973, Plate 3, Fig. 2) is not credible and seems extremely questionable. However, we cannot completely exclude the possibility of terminological confusion in the aforementioned article, the author of which did not indicate at what stages of the life cycle of Volvox she took material for research. The cells of the dividing embryo (not yet differentiated into two cell types) in this publication could be erroneously called somatic.
The first series of experimental studies on the senescence of somatic cells in Volvox was carried out on partially synchronized cultures of V. carteri f. nagariensis (Hagen and Kochert, 1980) and V. carteri f. weismannia (Pommerville and Kochert, 1981, 1982) at a temperature of 28–30°C, light-dark regimes 39 h : 9 h or 29 h : 19 h and transfers to fresh nutrient medium every two days (during these 48 h, one cycle of asexual development was completed). In these forms of Volvox, somatic cells from the moment of their formation to the stages of terminal differentiation and senescence in most cases increase in diameter from about 2 to 5–6 μm. In the above-mentioned studies of the laboratory of G. Kochert, significant differences were shown in the patterns of labeling of polypeptides (by 35S incorporation) between young somatic cells and gonidia, as well as between young and senescing somatic cells. These data indicated that V. carteri is promising as a model organism for studying the senescence process of the soma. It is appropriate to note that earlier in the same laboratory a method for separating two cell types in Volvox cultures for subsequent biochemical analysis was developed (Yates et al., 1975).
The somatic cells of young growing spheroids (after their release from the parental individual) are characterized by the intensive work of their flagella and actively synthesize the extracellular glycoproteinaceous matrix. At this step of development, protein synthesis in relatively young somatic cells proceeds about five times more intensively than in the aging hulk of the parental soma, inside which there are no longer any individuals of the young generation and which is not very motile due to a sharp weakening of the flagella activity (Hagen and Kochert, 1980; Pommerville, Kochert, 1982). However, even at this final step of the asexual life cycle, the somatic cells retain the ability to flagella regeneration, which takes two hours (deflagellation at both stages of development was performed by a sharp decrease in pH from 8.0 to 4.0 for 90 seconds) (Coggin and Kochert, 1986). Comparison of the characteristics of somatic cells at different stages of the cycle of asexual development shows that during senescence, along with a decrease in the content of proteins in cells and a decrease in the number of cytoplasmic ribosomes, there is also an intense accumulation of cytoplasmic lipid bodies, a slowdown in photosynthetic activity and then its loss, a disorganization of the chloroplast structure and a decrease in the chlorophyll content. As a result, the green pigmentation of cells weakens and eventually disappears (Hagen and Kochert, 1980; Pommerville and Kochert, 1981, 1982). These age-related changes in Volvox soma are gradual and cumulative; they resemble the processes occurring, for example, during leaf senescence in higher plants (Guo and Gan, 2005).
Unfortunately, a clear chronological sequence of the development of the soma, as well as unambiguous definitions of young, mature, and aging somatic cells, are absent in the works of Kochert’s laboratory. However, it has been shown that in V. carteri f. nagariensis somatic cells begin to senesce and die much earlier than in V. carteri f. weismannia (the lifetime of somatic cells after their initial formation during the development of a spheroid is about 4 and 5–7 days, respectively) (Pommerville and Kochert, 1982). The authors did not explain the reason for these differences in aging rates. Nonetheless, the data of Kochert’s pioneering studies suggested that the progress of Volvox somatic cells through the stages of senescence and death is a part of a genetically controlled endogenous developmental program, and not a passive process of necrosis. Instead of dying randomly, independently of each other, the somatic cells within one spheroid die synchronously. In addition, the loss of soma viability is significantly delayed when an aging Volvox colony is treated with a protein synthesis inhibitor cycloheximide at a concentration of 0.1 μg/mL. As a result, the authors (Pommerville and Kochert, 1982) concluded that the death of Volvox somatic cells is an active process. However, they did not explain what exactly they had in mind.
It is very interesting that in both forms of V. carteri, the somatic cells of female spheroids undergo aging much more slowly and die several days later than the somatic cells of asexual spheroids (Hagen and Kochert, 1980; Pommerville and Kochert, 1982). The authors again did not explain this result in any way. However, it seems to me possible to offer an appropriate commentary taking into account the specific traits of the cycle of sexual ontogenesis in Volvox. In this case, there is no release of young spheroids from the aging hulk of the parental soma. As a matter of fact, after the eggs contained in the female spheroid are fertilized, the resulting zygotes undergo a process of maturation that lasts several days inside the maternal individual. First, zygotes are dark green (light-dependent stage of ontogeny), and then red or orange and are surrounded by three envelopes (Starr, 1969; Kirk, 1998). Somatic cells die only after that. Thus, the cycle of sexual development in all Volvox species lasts significantly longer than the cycle of asexual development.
It is important that after the removal of gonidia from a young asexual spheroid of V. carteri f. nagariensis, the young hulk of somatic cells obtained in such an experiment will develop and senesce at the same rate as the control intact spheroids (Hagen and Kochert, 1980). On this basis, it was assumed that the development of reproductive cells does not affect the processes of terminal differentiation, senescence and death of somatic cells. However, this idea does not agree with the data of the same authors discussed above on the slowing down of the aging rate of somatic cells in the female spheroids (containing eggs instead of asexual gonidia). It would be very interesting to check the aging rate of the hulk of soma from the female spheroid after the experimental removal of eggs. Data on the absence of the influence of eggs on the aging process of somatic cells in a female individual of Volvox would give reason to assume that there might be different aging and death programs in soma from asexual and female spheroids. In addition, the idea was put forward (Kirk, 1998), which admitted in principal possibility that the rate of aging in the V. carteri soma could be influenced by the sex pheromone already mentioned above, which would then control the processes of ontogenesis not only in reproductive cells, but also in somatic cells.
In connection with the above data on the slow aging rates of somatic cells in the female V. carteri spheroids, it seems reasonable to mention the features of spheroid aging in several V. aureus strains. The fact is that this species is represented not only by strains, which are characterized by sexual reproduction and the formation of zygotes (Darden, 1966; Starr and Zeikus, 1993), but also by strains in which male spheroids are absent or extremely rare (Darden, 1968; Starr and Zeikus, 1993; Desnitski, 2000). In cultures of such strains, many gonidia are gradually transformed without fertilization into presumably haploid parthenospores (no cytological or genetic analysis was performed), although they have the same appearance as mature diploid zygotes and are also a resistant dormant stage. V. aureus spheroids containing parthenospores live several days longer than spheroids with gonidia (Desnitskiy, 2009). Thus, the parthenospore strains of this Volvox species could be used to analyze the characteristics of aging of their somatic cells.
Unfortunately, the studies of the Kochert laboratory on the senescence of somatic cells in V. carteri, considered in detail above, were not continued by that researcher. Let’s move on to the data of other authors, although the study of aging and death has not always been the main topic of their publications. A detailed microspectrofluorometric study (DAPI staining) of A. Coleman laboratory (Coleman and Maguire, 1982) showed that in V. aureus and V. carteri f. nagariensis, the content of nuclear DNA in somatic cells during the asexual development cycle remains constant from the moment of the embryonic cleavage completing until their death. In both Volvox species, the content of chloroplast DNA in somatic cells also does not change significantly during differentiation and aging (in contrast to a sharp increase in the content of chloroplast DNA in growing gonidia).
In the molecular genetic studies of D. Kirk’s laboratory, 12 transcripts specific for somatic cells and 19 transcripts specific for V. carteri f. nagariensis gonidia were identified (Tam and Kirk, 1991; Tam et al., 1991). These 12 somatic transcripts are divided into two categories: 5 “early” ones, which are actively accumulated starting from the moment of somatic cell formation immediately after cleavage of the embryo, and 7 “late” ones, which cannot be detected until young spheroids emerge from the old parental individual. However, the authors (Tam et al., 1991) doubted that “late somatic genes” play a decisive role in the aging of the soma, since the period during which there was a maximum accumulation of late transcripts precedes the aging phase. Interestingly, one of these late somatic genes (LSG2 gene) encodes a glycoproteinaceous lytic enzyme that accumulates in the extracellular glycoproteinaceous matrix of the developing spheroid. Later this enzyme takes part in the degradation (partial digestion) of the matrix, which will allow the release of a new generation of spheroids (Nishimura et al., 2017). Thus, the expression of the LSG2 gene indirectly prepares the basis for the subsequent aging of the somatic cell hulk.
Nevertheless, there are publications (Shimizu et al., 2001, 2002) showing a more important role of some other late somatic genes in V. carteri f. nagariensis during the aging process of the soma. For example, the gene encoding the aging-related RNase (ribonuclease) was cloned and studied. The corresponding mRNA is accumulated directly in the aging phase of somatic cells, while the content of total cellular RNA decreases many times during this process. Obviously, such results do not agree with the ideas of Kirk and co-authors (Tam and Kirk, 1991; Tam et al., 1991) about the role of late somatic genes in aging and death of the soma.
Finally, the data on type I and type II metacaspases in V. carteri f. nagariensis (Nedelcu, 2009b), proteolytic enzymes that play a key role in the process of programmed cell death (PCD) in lower eukaryotes (Bidle, 2016; Durand et al., 2016), deserve mention.
Thus, after reviewing the literature on the aging process and the subsequent death of somatic cells in Volvox, it becomes clear that this issue (despite its relevance) has not been sufficiently studied to date. In the literature on the ontogeny of Volvox, it has been repeatedly noted that its somatic cells undergo PCD (for example, Hagen and Kochert, 1980; Kirk et al., 1987; Kirk, 1998). However, the authors of one of the most recent reviews on the developmental biology of this green alga noted that they refrain from using the term PCD “since it implies a stereotyped set of events as defined by the process of apoptosis in animal cells that have not been examined in Volvox” (Matt and Umen, 2016, p. 109).
We will now proceed to a topic that usually does not attract the attention of researchers: the peculiarities of the death of gonidia in Volvox. More than a hundred years ago, A. Weismann (1904, p. 261) noted that gonidia (asexual reproductive cells that give rise to a new generation of Volvox spheroids) are potentially immortal. However, we must not forget that if resources are limited (both under natural conditions and under conditions of aging laboratory cultures), gonidia can die without giving offspring.
In connection with the above, an article is of interest, containing some information on asexual reproductive cells in an overcrowded aging culture of V. carteri f. nagariensis (Gilles and Jaenicke, 1982). While in an actively growing culture (with a density of about 10 spheroids per mL) spheroids usually contained 12–16 gonidia, in an aging culture (with a density of more than 1000 spheroids per mL) most spheroids contained 8–12 gonidia. My long-term observations on cultures of the same species and forma of Volvox are in accord with the data of the work cited above with respect to the number of gonidia in asexual spheroids from young and old cultures. Moreover, dealing with the Volvox cultures for a number of years, I observed that in aging cultures of V. carteri f. nagariensis (2–3 weeks after the transfer to a fresh nutrient medium) gonidia cease to enter the cleavage period, then gradually discolor and eventually die, sharing the fate of somatic cells. (It should be noted that in the collections of algae, Volvox is usually maintained by regular transfers to the fresh medium twice a month.)
In actively growing (young) cultures of V. aureus, asexual spheroids contain 3–15 gonidia (usually 8–12) (Darden, 1966; Shelton et al., 2012; etc.). On the other hand, my long-term observations have shown that in old cultures of V. aureus (approximately 3–4 weeks after transfer to the fresh medium), diminutive asexual spheroids are formed, containing only 1–3 gonidia. Thus, in this case, there is no cease of the process of asexual reproduction that occurs in the old cultures of V. carteri f. nagariensis. However, these old V. aureus cultures are not overcrowded that suggests the death of a significant part of the population along with the formation of a relatively small number of diminutive individuals, which can subsequently give normal offspring after transfer to the fresh medium. In this regard, it is appropriate to recall the publications that develop the concept of “altruistic” cell death in some unicellular protists in stressful situations (Durand et al., 2011, 2014; Nedelcu et al., 2011), when part of the population dies, supporting survival and reproduction of other individuals. However, at present it is difficult to assess how my previously mentioned preliminary and unpublished data on aging V. aureus cultures fit into the framework of the concept of altruistic cell death. In the future, it would be of interest to study this issue in more detail.
Thus, V. aureus and V. carteri f. nagariensis are characterized not only by different reproductive strategies under optimal cultivation conditions (as indicated above in the section on the development and cell differentiation in these algae), but also by different survival strategies if resources are limited in the aging cultures. In V. carteri f. nagariensis, the gonidial cleavage is blocked in such cultures, but for a relatively short time (several days) all reproductive cells retain the ability to start a new series of divisions (if conditions improve). In V. aureus, the ability to form new colonies retains much longer under unfavorable conditions (more than a month), but asexual reproduction occurs only in a relatively small number of individuals. This is in accord with my research on the ecology of Volvox (Desnitskiy, 2016, 2017, 2020), which shows that V. aureus, the only cosmopolitan and most frequently found in the wild representative of this genus of algae, is better adapted to life in sub-optimal and sub-extreme conditions than other species.
CONCLUSIONS
Volvox has become a promising model object, which is successfully used to study the processes of differentiation and morphogenesis in a relatively simple system consisting of only two types of cells. However, the present review of the data on senescence and cell death in this green alga shows that this topic is currently very poorly developed and further research is desirable. First of all, it would be important to clearly define and describe in detail the different stages of aging of the soma both during the asexual development cycle and in the ontogeny of colonies that form resistant dormant stages (zygotes or parthenospores). Also, a more detailed analysis of the aging process of laboratory cultures of Volvox and the death of gonidia that occurs when nutrient resources are limited is noteworthy.
The Volvox genus is characterized by a wide variety of developmental cycles. Therefore, in the future, for the experimental analysis of senescence and cell death, it would be optimal to apply a comparative approach and use not only V. carteri (the most studied species), but also other species (primarily V. aureus).
REFERENCES
Bhatia-Dey, N., Kanherkar, R.R., Stair, S.E., et al., Cellular senescence as the causal nexus of aging, Front. Genet., 2016, vol. 7, p. 13. https://doi.org/10.3389/fgene.2016.00013
Bidle, K.D., Programmed cell death in unicellular phytoplankton, Curr. Biol., 2016, vol. 26, no. 13, pp. R594–R607.
Brusentsev, E.Y., Tikhonova, M.A., Herbeck, Y.E., et al., Developmental aspects of senescence, Russ. J. Dev. Biol., 2017, vol. 48, no. 2, pp. 93–105.
Cheng, Q., Pappas, V., Hallmann, A., et al., Hsp70A and GlsA interact as partner chaperones to regulate asymmetric division in Volvox, Dev. Biol., 2005, vol. 286, no. 2, pp. 537–548.
Choi, G., Przybylska, M., and Straus, D., Three abundant germ line-specific transcripts in Volvox carteri encode photosynthetic proteins, Curr. Genet., 1996, vol. 30, no. 4, pp. 347–355.
Coggin, S.J. and Kochert, G., Flagellar development and regeneration in Volvox carteri (Chlorophyta), J. Phycol., 1986, vol. 22, no. 3, pp. 370–381.
Coleman, A.W., A comparative analysis of the Volvocaceae (Chlorophyta), J. Phycol., 2012, vol. 48, no. 3, pp. 491–513.
Coleman, A.W. and Maguire, M.J., A microspectrofluorometric analysis of nuclear and chloroplast DNA in Volvox, Dev. Biol., 1982, vol. 94, no. 2, pp. 441–450.
Darden, W.H., Sexual differentiation in Volvox aureus, Protozoology, 1966, vol. 13, no. 2, pp. 239–255.
Darden, W.H., Production of male-inducing hormone by a parthenosporic Volvox aureus, Protozoology, 1968, vol. 15, no. 3, pp. 412–414.
Davaapil, H., Brockes, J.P., and Yun, M.H., Conserved and novel functions of programmed cellular senescence during vertebrate development, Development, 2017, vol. 144, no. 1, pp. 106–114.
Deponte, M., Programmed cell death in protists, Biochim. Biophys. Acta, 2008, vol. 1783, no. 7, pp. 1396–1405.
Desnitski, A.G., Cellular mechanisms of the evolution of ontogenesis in Volvox, Arch. Protistenkd., 1992, vol. 141, no. 3, pp. 171–178.
Desnitski, A.G., A review on the evolution of development in Volvox—morphological and physiological aspects, Eur. J. Protistol., 1995, vol. 31, no. 3, pp. 241–247.
Desnitski, A.G., Development and reproduction of two species of the genus Volvox in a shallow temporary pool, Protistology, 2000, vol. 1, no. 4, pp. 195–198.
Desnitskiy, A.G., Volvox (Chlorophyta, Volvocales) as a model organism in developmental biology, Russ. J. Dev. Biol., 2009, vol. 40, no. 4, pp. 238–241.
Desnitskiy, A.G., Major ontogenetic transitions during Volvox (Chlorophyta) evolution: when and where might they have occurred?, Dev. Genes Evol., 2016, vol. 226, no. 5, pp. 349–354.
Desnitskiy, A.G., On ecological aspects of the evolutionary reorganizations of Volvox ontogeny, Int. J. Plant Reprod. Biol., 2017, vol. 9, no. 2, pp. 183–186.
Desnitskiy, A.G., Comparative analysis of embryonic inversion in algae of the genus Volvox (Volvocales, Chlorophyta), Russ. J. Dev. Biol., 2018, vol. 49, no. 3, pp. 129–133.
Desnitskiy, A.G., Advances in the research of sexual reproduction in colonial volvocine algae, Russ. J. Dev. Biol., 2019, vol. 50, no. 5, pp. 225–229.
Desnitskiy, A.G., Algae of the genus Volvox (Chlorophyta) in sub-extreme habitats, Int. J. Plant Reprod. Biol., 2020, vol. 12, no. 2, pp. 156–158.
Desnitskiy, A.G., Sym, S., and Durand, P.M., On the contribution of Mary Agard Pocock to developmental biology research of the genus Volvox L., Trans. R. Soc. S. Afr., 2018, vol. 73, no. 3, pp. 277–282.
van Doorn, W.G. and Woltering, E.J., Senescence and programmed cell death: substance or semantics?, J. Exp. Bot., 2004, vol. 55, no. 406, pp. 2147–2153.
Doronina, T.V., Sheval, E.V., and Lazareva, E.M., Programmed cell death during the formation of embryo sac and seed, Russ. J. Dev. Biol., 2020, vol. 51, no. 3, pp. 135–147.
Duncan, L., Nishii, I., Harryman, A., et al., The VARL gene family and the evolutionary origins of the master cell-type regulatory gene, regA, in Volvox carteri, J. Mol. Evol., 2007, vol. 65, no. 1, pp. 1–11.
Durand, P.M., Choudhury, R., Rashidi, A., et al., Programmed death in a unicellular organism has species-specific fitness effects, Biol. Lett., 2014, vol. 10, no. 2. https://doi.org/10.1098/rsbl.2013.1088
Durand, P.M., Rashidi, A., and Michod, R.E., How an organism dies affects the fitness of its neighbors, Am. Nat., 2011, vol. 177, no. 2, pp. 224–232.
Durand, P.M., Sym, S., and Michod, R.E., Programmed cell death and complexity in microbial systems, Curr. Biol., 2016, vol. 26, no. 13, pp. R587–R593.
Franklin, D.J., Brussaard, C.P.D., and Berges, J.A., What is the role and nature of programmed cell death in phytoplankton ecology?, Eur. J. Phycol., 2006, vol. 41, no. 1, pp. 1–14.
Gilles, R. and Jaenicke, L., Differentiation in Volvox carteri: study of pattern variation of reproductive cells, Zeitschr. Naturforsch., 1982, vol. 37, no. 10, pp. 1023–1030.
Goldsmith, T.C., Is the evolutionary programmed/non-programmed aging argument moot?, Curr. Aging Sci., 2015, vol. 8, no. 1, pp. 41–45.
Golstein, P., Aubry, L., and Levraud, J.-P., Cell-death alternative model organisms: why and which?, Nat. Rev. Mol. Cell Biol., 2003, vol. 4, no. 10, pp. 798–807.
Gordeeva, A.V., Labas, Y.A., and Zvyagilskaya, R.A., Apoptosis in unicellular organisms: mechanisms and evolution, Biochemistry (Moscow), 2004, vol. 69, no. 10, p. 1055–1066.
Grochau-Wright, Z.I., Hanschen, E.R., Ferris, P.J., et al., Genetic basis for soma is present in undifferentiated volvocine green algae, J. Evol. Biol., 2017, vol. 30, no. 6, pp. 1205–1218.
Guo, Y. and Gan, S., Leaf senescence: signals, execution, and regulation, Curr. Top. Dev. Biol., 2005, vol. 71, pp. 83–112.
Guzman-Lopez, J.A., Abraham-Juarez, M.J., Lozano-Sotomayor, P., et al., Arabidopsis thaliana gonidialess A/Zuotin related factors (GlsA/ZRF) are essential for maintenance of meristem integrity, Plant. Mol. Biol., 2016, vol. 91, nos. 1–2, pp. 37–51.
Hagen, G. and Kochert, G., Protein synthesis in a new system for the study of senescence, Exp. Cell Res., 1980, vol. 127, no. 2, pp. 451–457.
Hallmann, A., Evolution of reproductive development in the volvocine algae, Sex. Plant Reprod., 2011, vol. 24, no. 2, pp. 97–112.
Hanschen, E.R., Ferris, P.J., and Michod, R.E., Early evolution of the genetic basis for soma in the Volvocaceae, Evolution, 2014, vol. 68, no. 7, pp. 2014–2025.
Herron, M.D., Origins of multicellular complexity: Volvox and the volvocine algae, Mol. Ecol., 2016, vol. 25, no. 6, pp. 1213–1223.
Herron, M.D. and Michod, R.E., Evolution of complexity in the volvocine algae: transitions in individuality through Darwin’s eye, Evolution, 2008, vol. 62, no. 2, pp. 436–451.
Herron, M.D. and Nedelcu, A.M., Volvocine algae: from simple to complex multicellularity, in Evolutionary Transitions to Multicellular Life, Ruiz-Trillo, I. and Nedelcu, A.M., Eds., Dordrecht, The Netherlands: Springer, 2015, pp. 129–152.
Herron, M.D., Desnitskiy, A.G., and Michod, R.E., Evolution of developmental programs in Volvox (Chlorophyta), J. Phycol., 2010, vol. 46, no. 2, pp. 316–324.
Hoops, H.J., Nishii, I., and Kirk, D.L., Cytoplasmic bridges in Volvox and its relatives, in Cell–Cell Channels, Baluska, F., Volkmann, D., and Barlow, P., Eds., Georgetown (Texas): Landes Bioscience, 2006, pp. 65–84.
Igawa, T., Hoshino, Y., and Yanagawa, Y., Isolation and characterization of the plant glsA promoter from Alstroemeria, Plant Biol., 2009, vol. 11, no. 6, pp. 878–885.
Karn, R.C., Starr, R.C., and Hudock, G.A., Sexual and asexual differentiation in Volvox obversus (Shaw) Printz, strains WD3 and WD7, Arch. Protistenkd., 1974, vol. 116, nos. 1–2, pp. 142–148.
Kasuba, K.C., Vavilala, S.L., and D’Souza, J.S., Apoptosis-like cell death in unicellular photosynthetic organisms—a review, Algal Res., 2015, vol. 12, pp. 126–133.
Kirk, D.L., Volvox: Molecular-Genetic Origins of Multicellularity and Cellular Differentiation, New York: Cambridge Univ. Press, 1998.
Kirk, D.L., Germ-soma differentiation in Volvox, Dev. Biol., 2001, vol. 238, no. 2, pp. 213–223.
Kirk, D.L., A twelve-step program for evolving multicellularity and a division of labor, BioEssays, 2005, vol. 27, no. 3, pp. 299–310.
Kirk, D.L., Baran, G.J., Harper, J.F., et al., Stage-specific hypermutability of the regA locus of Volvox, a gene regulating the germ-soma dichotomy, Cell, 1987, vol. 48, no. 1, pp. 11–24.
Kirk, M.M., Ransick, A., McRae, S.E., et al., The relationship between cell size and cell fate in Volvox carteri, J. Cell Biol., 1993, vol. 123, no. 1, pp. 191–208.
Kirk, M.M., Stark, K., Miller, S.M., et al., regA, a Volvox gene that plays a central role in germ-soma differentiation, encodes a novel regulatory protein, Development, 1999, vol. 126, no. 4, pp. 639–647.
Kochert, G., Developmental mechanisms in Volvox reproduction, in The Developmental Biology of Reproduction, Markert, C.L. and Papaconstantinou, J., Eds., New York: San Francisco: Academic, 1975, pp. 55–90.
König, S.G. and Nedelcu, A.M., The genetic basis for the evolution of soma: mechanistic evidence for the co-option of a stress-induced gene into a developmental master regulator, Proc. R. Soc. London, Ser. B, 2020, vol. 287, article no. 20201414. https://doi.org/10.1098/rspb.2020.1414
Koufopanou, V., The evolution of soma in the Volvocales, Am. Nat., 1994, vol. 143, no. 5, pp. 907–931.
Koufopanou, V. and Bell, G., Soma and germ: an experimental approach using Volvox, Proc. R. Soc. London, Ser. B, 1993, vol. 254, no 1340, pp. 107–113.
Locato, V. and De Gara, L., Programmed cell death in plants: an overview, Methods Mol. Biol., 2018, vol. 1743, pp. 1–8.https://doi.org/10.1007/978-1-4939-7668-3_1
Matt, G. and Umen, J., Volvox: a simple algal model for embryogenesis, morphogenesis and cellular differentiation, Dev. Biol., 2016, vol. 419, no. 1, pp. 99–113.
Matt, G.Y. and Umen, J.G., Cell-type transcriptomes of the multicellular green alga Volvox carteri yield insights into the evolutionary origins of germ and somatic differentiation programs, G3 (Bethesda), 2018, vol. 8, no. 2, pp. 531–550.
Mori, T., Kuroiwa, H., Higashiyama, T., et al., Identification of higher plant glsa, a putative morphogenesis factor of gametic cells, Biochem. Biophys. Res. Commun., 2003, vol. 306, no. 2, pp. 564–569.
Nedelcu, A.M., Environmentally induced responses co-opted for reproductive altruism, Biol. Lett., 2009a, vol. 5, no. 6, pp. 805–808.
Nedelcu, A.M., Comparative genomics of phylogenetically diverse unicellular eukaryotes provide new insights into the genetic basis for the evolution of the programmed cell death machinery, J. Mol. Evol., 2009b, vol. 68, no. 3, pp. 256–268.
Nedelcu, A.M. and Michod, R.E., The evolutionary origin of an altruistic gene, Mol. Biol. Evol., 2006, vol. 23, no. 8, pp. 1460–1464.
Nedelcu, A.M., Driscoll, W.W., Durand, P.M., et al., On the paradigm of altruistic suicide in the unicellular world, Evolution, 2011, vol. 65, no. 1, pp. 3–20.
Nishimura, M., Nagashio, R., Sato, Y., et al., Late somatic gene 2 disrupts parental spheroids cooperatively with Volvox hatching enzyme A in Volvox, Planta, 2017, vol. 245, no. 1, pp. 183–192.
Nozaki, H., Morphology, sexual reproduction and taxonomy of Volvox carteri f. kawasakiensis f. nov. (Chlorophyta) from Japan, Phycologia, 1988, vol. 27, no. 2, pp. 209–220.
Nozaki, H., Matsuzaki, R., Yamamoto, K., et al., Delineating a new heterothallic species of Volvox (Volvocaceae, Chlorophyceae) using new strains of “Volvox africanus,” PLoS One, 2015, vol. 10. e0142632. https://doi.org/10.1371/journal.pone.0142632
Nozaki, H., Takusagawa, M., Matsuzaki, R., et al., Morphology, reproduction and taxonomy of Volvox dissipatrix (Chlorophyceae) from Thailand, with a description of Volvox zeikusii sp. nov., Phycologia, 2019, vol. 58, no. 2, pp. 192–199.
Nozaki, H., Mahakham, W., Heman, W., et al., A new preferentially outcrossing monoicous species of Volvox sect. Volvox (Chlorophyta) from Thailand, PLoS One, 2020, vol. 15, no. 7. e0235622. https://doi.org/10.1371/journal.pone.0235622
Pall, M.L., Mutants of Volvox showing premature cessation of division: evidence for a relationship between cell size and reproductive cell differentiation, in Developmental Biology: Pattern Formation, Gene Regulation, McMahon, D. and Fox, C.F., Eds., Menlo Park (California): W. A. Benjamin, Inc., 1975, pp. 148–156.
Pappas, V. and Miller, S.M., Functional analysis of the Volvox carteri asymmetric division protein GlsA, Mech. Dev., 2009, vol. 126, no. 10, pp. 842–851.
Pommerville, J.C. and Kochert, G., Changes in somatic cell structure during senescence of Volvox carteri, Eur. J. Cell Biol., 1981, vol. 24, no. 2, pp. 236–243.
Pommerville, J.C. and Kochert, G., Effects of senescence on somatic cell physiology in the green alga Volvox carteri, Exp. Cell Res., 1982, vol. 140, no. 1, pp. 39–45.
Rogers, H.J., Senescence-associated programmed cell death, in Plant Programmed Cell Death, Gunawardena, A.N. and McCabe, P.F., Eds., Cham (Switzerland): Springer, 2015, pp. 203–233.
Shelton, D.E., Desnitskiy, A.G., and Michod, R.E., Distribution of reproductive and somatic cell numbers in diverse Volvox (Chlorophyta) species, Evol. Ecol. Res., 2012, vol. 14, no. 6, pp. 707–721.
Shimizu, T., Inoue, T., and Shiraishi, H., A senescence-associated S-like RNase in the multicellular green alga Volvox carteri, Gene, 2001, vol. 274, nos. 1–2, pp. 227–235.
Shimizu, T., Inoue, T., and Shiraishi, H., Cloning and characterization of novel extensin-like cDNAs that are expressed during late somatic cell phase in the green alga Volvox carteri, Gene, 2002, vol. 284, nos. 1–2, pp. 179–187.
Skulachev, M.V. and Skulachev, V.P., Programmed aging of mammals: proof of concept and prospects of a biochemical approach for antiaging therapy, Biochemistry (Moscow), 2017, vol. 82, no. 12, pp. 1403–1422.
Soyer, M.-O., Complément á l'étude ultrastructural des Volvocales. Étude des colonies femelles de Volvox aureus E., Ann. Sci. Natur. Zool. (Ser. 12), 1973, vol. 15, no. 2, pp. 231–258.
Stark, K., Kirk, D.L., and Schmitt, R., Two enhancers and one silencer located in the introns of regA control somatic cell differentiation in Volvox carteri, Genes Dev., 2001, vol. 15, no. 11, pp. 1449–1460.
Starr, R.C., Structure, reproduction and differentiation in Volvox carteri f. nagariensis Iyengar, strains HK9 and 10, Arch. Protistenkd., 1969, vol. 111, nos. 3–4, pp. 204–222.
Starr, R.C., Control of differentiation in Volvox, Dev. Biol., 1970, suppl. 4, pp. 59–100.
Starr, R.C., Meiosis in Volvox carteri f. nagariensis, Arch. Protistenkd., 1975, vol. 117, nos. 1–2, pp. 187–191.
Starr, R.C. and Zeikus, J.A., Utex—the culture collection of algae at the university of Texas at Austin. 1993 list of cultures, J. Phycol., 1993, vol. 29, suppl. to no. 2, pp. 1–106.
Tam, L.-W. and Kirk, D.L., Identification of cell-type-specific genes of Volvox carteri and characterization of their expression during the asexual life cycle, Dev. Biol., 1991, vol. 145, no. 1, pp. 51–66.
Tam, L.-W., Stamer, K.A., and Kirk, D.L., Early and late gene expression programs in developing somatic cells of Volvox carteri, Dev. Biol., 1991, vol. 145, no. 1, pp. 67–76.
Umen, J.G., Volvox and volvocine green algae, EvoDevo, 2020, vol. 11, p. 13. https://doi.org/10.1186/s13227-020-00158-7
Weismann, A., The Germ-Plasm. A Theory of Heredity, New York: Charles Scribner’s Sons, 1893.
Weismann, A., The Evolution Theory, London: Edward Arnold, 1904, vol. 1.
Woo, H.R., Kim, H.J., Lim, P.O., et al., Leaf senescence: systems and dynamics aspects, Ann. Rev. Plant Biol., 2019, vol. 70, pp. 347–376.
Yates, I., Darley, M., and Kochert, G., Separation of cell types in synchronized cultures of Volvox carteri, Cytobios, 1975, vol. 12, nos. 47–48, pp. 211–223.
Zhu, Y., Liu, X., Ding, X., et al., Telomere and its role in the aging pathways: telomere shortening, cell senescence and mitochondria dysfunction, Biogerontology, 2019, vol. 20, no. 1, pp. 1–16.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
There is no conflict of interest.
This article does not contain any studies involving humans or animals performed by the author.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Desnitskiy, A.G. Volvox as a Model for Studying Cell Death and Senescence. Russ J Dev Biol 52, 259–267 (2021). https://doi.org/10.1134/S1062360421030036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360421030036