Abstract
The evolution of multicellularity, the separation of germline cells from sterile somatic cells, and the generation of a male–female dichotomy are certainly among the greatest innovations of eukaryotes. Remarkably, phylogenetic analysis suggests that the shift from simple to complex, differentiated multicellularity was not a unique progression in the evolution of life, but in fact a quite frequent event. The spheroidal green alga Volvox and its close relatives, the volvocine algae, span the full range of organizational complexity, from unicellular and colonial genera to multicellular genera with a full germ–soma division of labor and male–female dichotomy; thus, these algae are ideal model organisms for addressing fundamental issues related to the transition to multicellularity and for discovering universal rules that characterize this transition. Of all living species, Volvox carteri represents the simplest version of an immortal germline producing specialized somatic cells. This cellular specialization involved the emergence of mortality and the production of the first dead ancestors in the evolution of this lineage. Volvocine algae therefore exemplify the evolution of cellular cooperation from cellular autonomy. They also serve as a prime example of the evolution of complex traits by a few successive, small steps. Thus, we learn from volvocine algae that the evolutionary transition to complex, multicellular life is probably much easier to achieve than is commonly believed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the early work of August Weismann on the continuity of germ plasm at the end of the nineteenth century (Weismann 1893, 1892, 1889), the spheroidal green alga Volvox and its close relatives have been seen as suitable model organisms for addressing fundamental issues in the evolution of multicellularity and the development of a germ–soma dichotomy (Kirk 1998, 2001, 2003, 2005; Nozaki 2003; Hallmann 2003b; Schmitt 2003; Kirk and Kirk 2004; Michod et al. 2006). Volvox carteri illustrates the concept of a germ–soma dichotomy with diagrammatic clarity (Fig. 1): an adult V. carteri spheroid has only two cell types, ~2,000 small Chlamydomonas-like somatic cells, and ~16 large asexual reproductive cells (gonidia). The potentially immortal gonidia are nonmotile cells that are specialized for growth and reproduction; these cells constitute the germline. In contrast, the biflagellate somatic cells are specialized for extracellular matrix (ECM) biosynthesis, motility, and phototaxis. They are also incapable of dividing and are programmed to die when only a few days old. Therefore, of all living species, Volvox represents the simplest version of an immortal germline producing mortal soma.
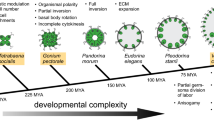
Rough approximation of the evolution of volvocine green algae from unicellular forms to colonial and multicellular forms with increasing complexity. Six representative species with characteristic developmental traits were arranged such that there is a progressive increase from left to right in morphologic and developmental complexity (Kirk 1998, 2000; Prochnik et al. 2010). Check marks indicate that a given trait is present in the respective species. Graded differences in a given trait are indicated by 1–5 plus signs and ± indicates ambiguity or occasional occurrence. The photomicrographs show Chlamydomonas reinhardtii (SAG 11-32b), Gonium pectorale (SAG 12.85), Pandorina morum (SAG 60-1d), Eudorina unicocca (SAG 24-1c), Pleodorina californica (SAG 32.94), and Volvox carteri (Eve). The exact phylogenetic position of these six species is indicated with filled black circles in the evolutionary tree in Fig. 3
Particularly with regard to questions about the evolution of multicellularity and of the germ–soma dichotomy, both Volvox and its close relatives, the volvocine green algae are of special interest (Kirk 1998, 2005; Prochnik et al. 2010). The volvocine green algae form a group of genera closely related to the genus Volvox within the order Volvocales (Chlorophyta). This group spans the full range of organismal complexity, from unicellular organisms, such as Chlamydomonas reinhardtii, to colonial organisms without a division of labor, such as Gonium pectorale, Pandorina morum, and Eudorina unicocca, to multicellular organisms with a partial or full germ–soma division of labor, such as Pleodorina californica and V. carteri, respectively (Fig. 1).
To facilitate detailed molecular analyses in volvocine algae, an array of important modern analytical tools has been developed. For example, stable nuclear transformation is possible (Fernández et al. 1989; Debuchy et al. 1989; Kindle et al. 1989; Mayfield and Kindle 1990; Schiedlmeier et al. 1994; Lerche and Hallmann 2009), unselectable markers can be efficiently co-transformed together with selectable markers (Kindle et al. 1991; Gruber et al. 1992; Schiedlmeier et al. 1994; Minko et al. 1999; Lerche and Hallmann 2009), suitable reporter genes and both inducible and constitutive promoters are available (Goldschmidt-Clermont 1991; Hallmann and Sumper 1994; Lerche and Hallmann 2009), expression of chimeric genes works (Blankenship and Kindle 1992; Hallmann and Sumper 1994; Lerche and Hallmann 2009), foreign genes can be expressed (Hallmann and Sumper 1996; Stevens et al. 1996; Hallmann and Rappel 1999; Lerche and Hallmann 2009), dominant selectable markers are available (Nelson et al. 1994; Hallmann and Rappel 1999), gene replacement by homologous recombination is feasible (Sodeinde and Kindle 1993; Hallmann et al. 1997), plasmids or transposons can be used for gene tagging (Tam and Lefebvre 1993; Schnell and Lefebvre 1993; Kirk et al. 1999; Miller and Kirk 1999; Ueki and Nishii 2008), and the genomes of C. reinhardtii and V. carteri have been sequenced (Merchant et al. 2007; Prochnik et al. 2010).
In summary, the existence of a germ–soma division of labor in Volvox, the range of complexity from unicellular to multicellular species in volvocine algae, and the availability of an extensive molecular toolkit make volvocine green algae useful model organisms for studying the evolution of multicellularity and of sterile somatic cells.
A phylogenetic perspective on the transition from unicellularity to multicellularity
In many textbooks, volvocine algae are used to illustrate the evolutionary transition from unicellular to multicellular organisms, and some readers might therefore mistakenly arrive at the conclusion that this transition happened only once in the evolution of all multicellular life. However, multicellularity has arisen independently again and again in the tree of life, over billions of years. In fact, these transitions were not even restricted to eukaryotes but occurred in prokaryotes as well: The first evidence of a shift to multicellularity comes from fossilized Cyanobacteria-like prokaryotes, which lived 3–3.5 billion years ago (Schopf 1993; Knoll 2003), and the first multicellular eukaryotes may have existed about 1 billion years ago (Knoll et al. 2006). Between about 530 and 570 million years ago, a burst of diversification occurred, with the eventual appearance of the lineages of almost all metazoa living today (Carroll 2001; Knoll 2003; King 2004; Maynard Smith and Szathmáry 1995; Pfeiffer and Bonhoeffer 2003; Grosberg and Strathmann 2007). Among the eukaryotes, multicellular organisms independently originated at least 25 times from unicellular ancestors (followed in some cases by secondary losses) (Buss 1987; Cavalier-Smith 1991; Maynard Smith and Szathmáry 1995; Kirk 1998; Bonner 1998, 2000; Carroll 2001; Kaiser 2001; Medina et al. 2003; Baldauf 2003; King 2004; Grosberg and Strathmann 2007; Rokas 2008; Prochnik et al. 2010). Almost every lineage of the eukaryotic tree of life includes multicellular forms: the most well-known lineages are the Embryophyta (land plants), Chlorophyta (green algae), Rhodophyta (red algae), Phaeophyceae (brown algae), Bacillariophyta (diatoms), fungi, and animals (Fig. 2).
Phylogenetic distribution of multicellularity among eukaryotes. Large U, only unicellular forms; large M, only colonial/multicellular forms; middle-sized U and M, clade with both unicellular and colonial/multicellular forms; large U and small M, mainly unicellular forms with rare colonial/multicellular forms (Baldauf 2003; King 2004; Grosberg and Strathmann 2007; Prochnik et al. 2010)
For most multicellular lineages, it has been quite challenging to investigate the shifts to multicellularity because the transitions occurred so long ago (Herron and Michod 2008). Therefore, a molecular understanding of the genetic changes underlying this transition might even be unattainable in these lineages. In contrast, the volvocine algae represent a unique opportunity to study the transition to multicellularity at a molecular level because the multicellular members of this group diverged relatively recently from their unicellular relatives: the last common ancestor of the unicellular alga C. reinhardtii and the multicellular alga V. carteri lived only 50–200 million years ago (Rausch et al. 1989; Herron and Michod 2008; Herron et al. 2009). Moreover, extant volvocine species display a range of intermediate grades between unicellular forms and multicellular forms with a complete separation of germ and soma (Fig. 1).
Taxonomically, the volvocine algae belong to the order Volvocales. Together with orders such as the Dunaliellales, Chlorococcales, Oedogoniales, and Chaetophorales, the Volvocales belong to the Chlorophyceae, one of the four classes of Chlorophyta (green algae) (Fig. 2). Detailed molecular phylogenetic analyses of the volvocine algae have revealed that the history of the whole group is quite complex, and not just a simple, linear progression in size and complexity (Nozaki et al. 2000; Nozaki 2003; Nakada et al. 2008; Herron and Michod 2008; Herron et al. 2010). Therefore, the few volvocine species arranged in order of increasing complexity in Fig. 1 reflect only a rough approximation of the evolution of volvocine green algae. The detailed molecular phylogenetic reconstruction of volvocine algae shown in Fig. 3 confirms that volvocine algae constitute a robust monophyletic group with two major families within this group, the Goniaceae and the Volvocaceae (Larson et al. 1992; Nozaki et al. 1995, 1999, 2000, 2002; Kirk 1998; Coleman 1999; Nozaki 2003; Nakada et al. 2008; Herron and Michod 2008; Herron et al. 2010). However, the evolutionary tree of volvocine algae is highly branched, and several taxa, including the genus Volvox, are found on more than one branch. The structure of volvocine taxonomy results from the fact that early classifications of this group were based on morphological correlations rather than phylogenetic relationships; several nominal taxa appear to represent convergent morphologies rather than monophyletic relationships. Therefore, the genus and species names of volvocine algae probably should be revised based on molecular phylogenetic reconstructions (Fig. 3).
Evolutionary tree of volvocine algae based on the nucleotide sequences of five chloroplast genes. This phylogenetic analysis indicates that multicellularity evolved only once in this group. In contrast, a partial germ–soma division of labor evolved independently in three different lineages and was lost twice (Nozaki et al. 1997; Herron and Michod 2008; Herron et al. 2009). A full germ–soma division also evolved three times. The meanings of symbols and letters are given in the figure. Names of strains are given in gray behind the species names. The species shown on the photomicrographs in Fig. 1 are indicated with filled black circles. This tree was adapted from Herron and Michod (2008) and others (Sachs 2008; Herron et al. 2010); some additional information was added (Lerche and Hallmann 2009; Ueki et al. 2010)
A detailed molecular phylogenetic analysis indicates that multicellularity evolved only once in volvocine algae (Fig. 3) (Larson et al. 1992; Nozaki et al. 1995, 1999, 2000, 2002; Kirk 1998; Coleman 1999; Nozaki 2003; Nakada et al. 2008; Herron and Michod 2008; Herron et al. 2010). After this transition to multicellularity, a partial germ–soma division of labor emerged independently in three different lineages. Organisms are characterized as exhibiting a “partial division of labor” if their gonidia are derived by redifferentiation of cells that had initially differentiated as biflagellate (somatic-like) cells and contributed to the organism’s motility, whereas organisms are characterized as exhibiting a “full” or “complete division of labor” if their gonidia differentiate without first developing functional flagella and contributing to the motility of the organism (Herron et al. 2010). In volvocine algae, a full germ–soma division of labor evolved three times (within one of the three lineages that show partial germ–soma division) and led to the species Volvox gigas, Volvox africanus, Volvox obversus, and V. carteri (Fig. 3) (Nozaki et al. 1997; Herron and Michod 2008; Herron et al. 2009, 2010). The evolution of germ–soma division also reflects the evolution of a sterile soma, which involves the emergence of mortality and the production of the first dead ancestors in the evolution of this lineage. Remarkably, the innovation “division of labor” was actually lost twice in volvocine algae (Nozaki et al. 1997; Herron and Michod 2008; Herron et al. 2009, 2010).
Driving forces in the evolutionary transition toward differentiated multicellularity
Many previous studies address the question why and how multicellularity and germ–soma differentiation evolved in volvocine algae (and elsewhere) (Bonner 1965, 1974, 1988, 1993, 1998, 2000, 2003; Kirk 1988, 1994, 1995, 1997, 1998, 1999, 2000, 2001, 2003, 2005; Kirk et al. 1990, 1993; Koufopanou and Bell 1991, 1993; Desnitski 1993, 1995; Koufopanou 1994; Bell 1998; Schmitt 2001, 2003; Miller 2002, 2010; Stark and Schmitt 2002; Hallmann 2002, 2003a, b, 2006b; Michod et al. 2003, 2006; Pfeiffer and Bonhoeffer 2003; Kirk and Kirk 2004; Nedelcu and Michod 2004; Cheng et al. 2005; Solari et al. 2006a, b, 2007; Grosberg and Strathmann 2007; Willensdorfer 2008, 2009; Sachs 2008; Rokas 2008; Herron et al. 2009; Gavrilets 2010). It appears to be a matter of course that there must be a combination of costs and benefits that accompany increased body size, such that under certain conditions the benefits of larger size outweigh its costs; it was argued that size increase came first, and the possible advantages that this change might provide would follow (Bonner 1998). One such benefit of size is predation, which spares larger organisms (see below) (Bell 1985; Kirk 1998). Following the origin of multicellularity in the above-mentioned groups of organisms (Fig. 2), e.g., land plants, green algae, red algae, brown algae, fungi, and animals, there evolved more than one cell type. It has been assumed that also early differentiation is related to the size of the organism: the larger the organism, the more cell types (Bonner 2003). Thus, size plays a critical role in influencing the degree of differentiation (Bonner 2003). But how can groups of cells become individuals? It was hypothesized that fitness trade-offs drive the transition of a cell group into a multicellular individual through the evolution of cells specialized at reproductive and vegetative functions of the group (Michod 2007). Somatic cells specialized at vegetative functions describe a reproductive altruism, which may have evolved through co-option of life-history trade-off genes present in a unicellular ancestor; the selective pressures leading to reproductive altruism in differentiated, multicellular volvocine algae obviously stem from the increasing cost of reproduction with increasing group size (Michod 2007).
Life cycles of the volvocine algae
Volvocine algae are able to reproduce both asexually and sexually (Figs. 4, 5, 6); however, in these algae, the principal mode of reproduction is asexual (Kirk 1998; Harris et al. 2009). The multicellular alga V. carteri is dioecious (i.e., it produces separate male and female organisms), but the asexual life cycles of both sexes (mating types) are indistinguishable (Figs. 5, 6); the same is true for the unicellular alga C. reinhardtii (Fig. 4) (Kirk 1998; Harris et al. 2009).
Volvocine algae switch from their principal, asexual mode of reproduction to a sexual mode when life-threatening conditions approach (Figs. 4, 6) (Kirk 1998; Harris et al. 2009). Thus, sexuality is a form of emergency response program for volvocine algae. Although the sexual cycle results in the production of resistant zygospores, as well as genetic recombination and repair, it does not result in any significant amount of increase in population size.
Key evolutionary innovations in the asexual life cycle
When multicellular organisms evolved from unicellular ones, previous achievements had to be modified and developed to fulfill the requirements of multicellularity (Szathmáry and Smith 1995; Bonner 1998; Grosberg and Strathmann 2007; Prochnik et al. 2010). A comparison of the developmental and reproductive traits of the modern multicellular alga V. carteri with those of the extant unicellular alga C. reinhardtii and species with intermediate complexity provides insight into the evolutionary innovations that were required for multicellular life.
The cell division cycle of most asexually reproducing volvocine algae is different from the cycles seen in most other organisms (Kirk 2003, 1998). The asexual reproductive cells of most volvocine algae grow 2n-fold in size and then divide rapidly n times by multiple fission to produce 2n offspring cells. This type of cell division is known as palintomy and multiple fission (Sleigh 1989; Desnitski 1995). The n has a value from 2 to 15 depending on the volvocine species and, to some extent, the environmental conditions (Kirk 2003, 1998). In C. reinhardtii, the n is usually 2 or 3 (Fig. 4a, b), and in V. carteri, it is usually 11 or 12 (Fig. 5). However, there is an important difference between the multiple fission programs of these two species: the offspring cells in C. reinhardtii almost always separate from each other (complete cytokinesis) and eventually become unicellular individuals, while the offspring cells in multicellular volvocine algae stay linked to each other by cytoplasmic bridges throughout the rest of embryogenesis due to an incomplete cytokinesis (Fig. 1). Hence, incomplete cytokinesis and an increase in the number of cleavage divisions led to an evolutionary rise in adult cell number in volvocine algae, ranging from one cell in C. reinhardtii to many thousands of cells in various species of Volvox (Figs. 1, 6a, b).
Asexual and sexual reproduction in C. reinhardtii. Under favorable conditions of growth, Chlamydomonas reproduces asexually; only when the conditions are unfavorable does it reproduce sexually. a and b During asexual reproduction, cells grow and undergo two or more rounds of mitosis and cytokinesis before the daughter cells hatch from the old cell wall. Both mating types, the plus mating type (mt+) (a) and the minus mating type (mt−) (b), show identical asexual life cycles. c Sexual reproduction is induced by unfavorable conditions (nitrogen limitation), and asexual cells of both mating types develop into gametes. Gametes of opposite mating types are able to form aggregates via flagellar agglutination. After the release of cell walls, mating structures are formed, and the gametes fuse (plasmogamy and karyogamy) to form a zygote. The zygote further matures into a dormant, heavily walled, and diploid zygospore. At the time of spore germination, meiosis occurs to form four haploid progeny cells (two of each mating type), each of which grows into an asexual cell of the respective mating type
The asexual life cycle of V. carteri. Asexual development in males and females is indistinguishable. During embryogenesis, mature asexual reproductive cells (gonidia) undergo a rapid series of 11–12 cleavage divisions, some of which are asymmetric. The larger cells resulting from these unequal divisions will become the gonidia of the next generation, whereas the smaller cells will become part of the somatic cell population. At the end of cleavage, the embryo is inside out with respect to the adult configuration: its gonidia are on the outside, and the flagella of its somatic cells are pointing toward the interior of the sphere. A morphogenetic process called inversion brings the embryo into its adult configuration through a series of cell movements that resemble gastrulation in animal embryos (Kirk et al. 1982). The juveniles expand by the deposition of ECM and then hatch from their parent spheroid during a process called release. The somatic cells of the parent, lacking reproductive cells and thus incapable of further cleavage, undergo senescence and die, while the gonidia of the juvenile spheroids mature. Under standard conditions (Starr 1969), the asexual life cycle takes 48 h. For clarity, only 4 of the ~16 gonidia/embryos/descendant spheroids are shown within each parent spheroid. Photomicrographs of asexual males and females are shown in Fig. 6a, b
The switch from asexual to sexual reproduction in V. carteri. Asexual development (see Fig. 5) and the phenotypes of males and females are indistinguishable. a Photomicrograph of an asexual male. b Photomicrograph of an asexual female. When the sex inducer is present, the gonidia of both sexes undergo a modified embryogenesis, which is also different in males and females. c In males, embryonic cleavage divisions (including asymmetric cell division) result in juveniles with somatic cells and sperm packets in a 1:1 ratio. d In females, juveniles with ~32 eggs arise. Mature sperm packets (e) are released and swim around until they contact a sexual female with mature eggs. The sperm packets then break up into individual sperm, and the sperm penetrate the ECM of the female and fertilize the eggs (f). g The resulting diploid zygotes develop an orange coloration and secrete a thick, crenellated cell wall. When the zygote germinates, it undergoes meiosis to produce either a single haploid asexual male or female
At the end of cleavage, each embryo of colonial or multicellular volvocine algae contains all of the cells that will be present in an adult of the next generation. However, the orientation of the cells of the embryo is inside out with respect to the adult configuration; that is, the cells have their flagellar ends pointing toward the interior, rather than toward the exterior where they will be needed to function in locomotion. To correct this maladaptive arrangement and to achieve the adult configuration, the embryo turns itself right-side out in a gastrulation-like morphogenetic process called (embryonic) inversion (Figs. 1, 5) (Hallmann 2006a). The cytoplasmic bridges that link all embryonic cells play a major role in inversion (Kirk 1998; Kirk and Nishii 2001).
The shift to organisms with more than one cell also required the elaboration of a multifunctional ECM out of the simple ECM (usually called cell wall) of the Chlamydomonas-like ancestor. In all volvocine algae, the structured ECM meshwork is assembled mainly from hydroxyproline-rich glycoproteins (Miller et al. 1974; Kirk et al. 1986; Sumper and Hallmann 1998; Hallmann 2003b, 2006b). In volvocine algae with high levels of organismal complexity like Volvox, the ECM not only embeds the cells in the surface of a transparent sphere, holds them together and allows for their suitable orientations but also represents a rather dynamic and multifunctional interface between each cell and its neighboring cells and/or environment. The ECM is continuously adapted to changing intracellular, organism-specific or environmental conditions, and ECM remodeling is essential for embryonic development, morphogenesis, and reproduction (Nagase and Woessner 1999; Hallmann 2003b). As the number of cells increased during the evolution of the volvocine lineage, the size of organisms increased even more rapidly due to an exponential increase in the volume of ECM per cell (Fig. 1). In adult V. carteri spheroids in the stage shortly before the release of juveniles (Fig. 5), the ECM constitutes up to 99% of the spheroid volume, whereas C. reinhardtii has only a relatively thin ECM (Fig. 1). The evolution of large multicellular spheroids with a voluminous ECM might result in an improved protection of the next generation, which develops inside the parent spheroid (Fig. 5). Additionally, predation spares larger organisms: small green flagellates are subject to predation by invertebrate filter feeders such as rotifers and small crustaceans, but most colonial volvocine algae are too large to be consumed by these filter feeders (Bell 1985; Kirk 1998).
Traits that impact forward swimming and phototaxis also required evolutionary adaptation during the transition to an organism with more than one cell. In multicellular organisms, phototactic swimming requires a proper and stable orientation of cells with respect to one other and some kind of coordination between cells (Ueki et al. 2010). Furthermore, unicellular volvocine algae like Chlamydomonas show a characteristic breaststroke-type swimming motion, which is inappropriate for propelling a multicellular spheroidal organism. In multicellular volvocine algae, the effective strokes of both flagella of each cell beat in more or less the same direction to push the organism forward (Gerisch 1959; Hoops and Floyd 1983; Hoops 1993; Ueki et al. 2010). This change in beating was accomplished by an evolutionary rotation of the basal bodies, which underlie the flagella and determine their orientations (Fig. 1) (Gerisch 1959; Greuel and Floyd 1985; Kirk 2005). In multicellular volvocine algae, flagellar beating also evolved in such a way that it results in rotation of the spheroid instead of rotation of the cell, because even in the multicellular species, the simple eyespot of each cell, a primitive visual system, is used like radar to scan the environment for light sources.
The evolutionary adjustment of flagellar beating direction in colonial and multicellular volvocine species also coincided with the invention of organismal polarity and an anterior–posterior axis, which is defined by the direction of swimming (Fig. 1). The colonial and multicellular algae swim in a posterior-to-anterior direction because their flagella beat in an anterior-to-posterior direction. In volvocine algae with increased organismal complexity, there is also a gradient in cell size along the anterior–posterior axis, with the largest cells at the anterior pole and the smallest at the posterior pole (Kirk 1998; Ueki et al. 2010). The cell-to-cell distances at the anterior pole are also larger than at the posterior pole. In volvocine species with a germ–soma division of labor, the reproductive cells are normally located within the posterior hemisphere. Another sign of organismal polarity is a gradient in eyespot size, with cells at the anterior pole having the largest and most light-sensitive eyespots; at the posterior pole, the eyespots are tiny or absent, making the corresponding cells appear to be blind (Ueki et al. 2010). The differences in eyespot size produce a photoresponsive gradient decreasing from the anterior to the posterior pole of the spheroid, with the highest responses at the anterior and no response at the posterior. The differences in cell and eyespot sizes reflect functional differences between previously identical cells and thus the evolution of specialization.
The transition to differentiated multicellularity also greatly affected reproductive traits. In C. reinhardtii (Figs. 1, 4) and other less-complex volvocine algae like G. pectorale and P. morum (Fig. 1), there is only one cell type. For asexual reproduction, the biflagellate cells grow, redifferentiate as nonmotile cells, and then undergo multiple fission to form daughter colonies. Once all cells of these daughter colonies have developed flagella, the daughters hatch and swim away.
In volvocine algae with an intermediate level of organismal complexity namely, certain species and strains of Eudorina, a few of the cells at the anterior pole of the embryo retain their flagella and fail to divide. In other words, the first sterile somatic cells emerged in evolution of this lineage (Fig. 1). The formation of sterile somatic cells is also graded along the anterior–posterior axis, with the sterile cells at the anterior pole (Kirk 2005).
The first clear sign of cell differentiation and germ–soma dichotomy in the volvocine lineage becomes apparent in P. californica (Fig. 1; Gerisch 1959). This alga shows a partial germ–soma division of labor: all cells initially differentiate into biflagellate cells, but then only cells of the posterior hemisphere redifferentiate as gonidia, while the cells of the anterior hemisphere remain as sterile somatic cells and eventually die.
In V. carteri, cell differentiation and germ–soma dichotomy have been fully established, i.e., this alga shows a complete germ–soma division of labor between ~2,000 somatic cells and ~16 germline cells (Fig. 1). During embryogenesis of volvocine species with a complete germ–soma division of labor, some of the cleavage divisions are asymmetric, producing large–small sister-cell pairs (Fig. 1) (Kirk 1995, 1998). In V. carteri, asymmetric cell division is accompanied by a bifurcation of the cell division program (Fig. 1; Kirk 2005): V. carteri embryos first cleave symmetrically five times to form a 32-cell embryo with identical cells, and then 16 cells divide asymmetrically to produce one large gonidial cell initial and one small somatic cell initial each (Kirk and Kirk 2004). These gonidial initials divide asymmetrically two more times and produce additional somatic initials at each division. The gonidial initials then temporarily stop any cleavage activity, while the somatic initials divide symmetrically about three more times. At the end of embryogenesis in V. carteri, the volume of the gonidial initials is about 30-fold larger than that of the somatic initials (Figs. 1, 7); however, the cells are only different in size. Then, by an as-yet-unknown mechanism, the size of each sister cell leads to the activation of either a somatic or germline program (Fig. 7) (Kirk et al. 1993). Thus, small cells develop as biflagellate somatic cells for ECM biosynthesis, motility, and phototaxis, and large cells develop as nonmotile, germline gonidia specialized for growth and reproduction (Figs. 1, 5, 6a, b, 7; Kirk et al. 1993). The fact that the gonidia develop directly without first developing functional flagella is the criterion for “complete” germ–soma division of labor.
Minimal model for the genetic program of germ–soma differentiation in V. carteri. Three key gene types are involved in programming differentiation: glsA, lag, and regA. At the 32-cell stage, the glsA gene acts to promote the asymmetric divisions that produce large–small sister-cell pairs. The lag genes then act in the large cells to repress the development of somatic characteristics, while the regA gene acts in the small cells to repress reproductive development. Adapted from D. L. Kirk (Kirk 2001, 2005; Kirk and Kirk 2004)
Mutational analysis of the genetic program for germ–soma differentiation in V. carteri identified three types of key genes: gonidialess A (glsA), regenerator A (regA), and late gonidia (lag) genes. Based on the mutational and molecular genetic results, a minimal model for the genetic program of germ–soma differentiation was established by David Kirk (Fig. 7; Kirk 1998, 2001, 2005; Kirk and Kirk 2004). In this model, the glsA gene acts during cleavage to permit asymmetric division and production of the large–small sister-cell pairs (Miller and Kirk 1999). In the small (somatic) cells, the regA gene acts as a transcriptional repressor to prevent all aspects of reproductive development (e.g., by repressing chloroplast biogenesis), while somatic genes are transcribed (Meissner et al. 1999; Kirk et al. 1999). The lag genes act as transcriptional repressors in the large cells (gonidia) to prevent the formation of somatic characteristics such as flagella and eyespots, while gonidial genes are transcribed (Kirk 1998). It is likely that the difference in cell size at the end of cleavage determines whether regA or the lag genes will be activated. However, it remains unclear how cell size is signaled to the regulatory program, which other components are involved and how the identified key genes fit into a larger regulatory network.
Several paralogous genes that encode proteins resembling regA have been identified both in V. carteri [e.g., the regA-like sequence D (rlsD) gene] and C. reinhardtii [e.g., RLS1] (Kirk et al. 1999; Duncan et al. 2006, 2007). However, Chlamydomonas does not have a gene that is orthologous to regA (Duncan et al. 2007). The closest homolog of Volvox regA in C. reinhardtii is RLS1, but the orthologous gene of C. reinhardtii RLS1 in Volvox is rlsD (Nedelcu 2009). These findings might be an indication of gene duplications in the common unicellular ancestor of Volvox and Chlamydomonas and then divergence of one of the gene copies, regA. It was speculated that the ancestor of regA might have acted in a stress-activated pathway that led to the repression of growth and cell division in response to energy or nutrient deprivation (Nedelcu 2009; Miller 2010). The paralogous genes of regA both in Volvox and Chlamydomonas might still have this function, while the function of regA might have changed and then was used to co-opt the entire pathway to repress growth and division in a developmental context (Nedelcu 2009; Miller 2010). In contrast to the regA gene, the glsA gene of V. carteri has an orthologous gene in C. reinhardtii, GAR1 (Cheng et al. 2003). GAR1 was shown to function just like glsA: When transformed into Volvox glsA mutants, it rescued the wild-type phenotype (Cheng et al. 2003). In the evolution of the volvocine lineage, glsA obviously was adopted for its novel function in germ–soma differentiation with no significant changes.
Key evolutionary innovations in the sexual life cycle
In the case of C. reinhardtii, the usual trigger for the switch to the sexual mode of reproduction is a lack of nitrogen (Sager and Granick 1954), indicating nutrient deficiency; in the case of V. carteri, the transition can be triggered by heat shock (Kirk and Kirk 1986), indicating drying up of the pond. The sexual cycle produces heavily walled, dormant zygotes (zygospores) that can resist tough conditions like drought, heat, and cold for a long period of time. Volvocine algae are haplonts, and the only diploid stage of development is the zygote.
In the unicellular alga C. reinhardtii, haploid vegetative cells of both sexes, the plus and minus mating types, differentiate into gametes in response to nitrogen starvation, and the isogamous gametes of opposite mating types can agglutinate and fuse to form zygotes (Harris et al. 2009). The zygote further matures into a dormant zygospore. At the time of spore germination, meiosis occurs to form four haploid progeny cells, each of which is able to enter the asexual life cycle (Fig. 4).
In the multicellular alga V. carteri, heat shock causes the production and release of a sex-inducer glycoprotein (Starr 1970; Starr and Jaenicke 1974; Kirk and Kirk 1986; Tschochner et al. 1987; Mages et al. 1988), which is one of the most potent biological effector molecules known because it can act at concentrations as low as 10−16 M (Gilles et al. 1984; Starr 1970; Sumper et al. 1993). Reproductive cells of asexually grown individuals (Fig. 6a, b) that have been exposed to the sex-inducer switch to the sexual mode (Hallmann et al. 1998). In sexually induced female embryos, the first asymmetrical cell division is postponed from the sixth to the seventh division cycle (Starr 1969, 1970; Hallmann et al. 1998). After the asymmetric cell division, the somatic cell initials undergo further cleavage (as in asexual embryos). The large reproductive cell initials develop into eggs, such that the sexual female ends up with ~32 eggs and ~2,000 somatic cells (Fig. 6d), compared to ~16 gonidia and ~2,000 somatic cells in asexual females (Fig. 6b). Sexually induced male embryos exhibit yet another pattern of asymmetric division (Starr 1969, 1970; Hallmann et al. 1998): the asymmetric cell division is postponed from the sixth to the eighth division cycle, and thereafter, the somatic cell initials no longer divide. Then about a day later, the large reproductive cell initials each undergo a new round of up to seven symmetric divisions, to form sperm packets containing up to 128 sperm. The sexual male thus ends up with 128 sperm packets and 128 somatic cells (Fig. 6c), compared with ~16 gonidia and ~2,000 somatic cells in asexual males (Fig. 6a) (Starr 1969, 1970; Hallmann et al. 1998). Eventually, the sperm packets, consisting of biflagellate sperm (Fig. 6e), are released from the spheroid. As soon as a swimming sperm packet makes contact with a sexual female, which seems to happen by chance rather than by chemoattraction (Kirk 1998), the packets break up into individual sperm, and the sperm penetrate the ECM of the female to reach the eggs inside the sphere (Fig. 6f) and fertilize them. Following fertilization, the resulting diploid zygotes mature and build tough cell walls (Fig. 6g). After a rest period, favorable conditions cause the zygotes to undergo meiosis and germination to form only a single viable germling and three nonviable small polar bodies (Starr 1975). The germling will produce a haploid female or male, depending on its genetic sex, which then reproduces asexually.
The sexual dimorphism, i.e., male–female dichotomy and anisogamy (oogamy), observed in V. carteri (Fig. 6) and many other volvocine species with an increased level of organismal complexity (Fig. 1) evolved from a Chlamydomonas-like, isogamous unicellular ancestor. Phylogenetic analyses indicate that either oogamy evolved twice or it evolved once and was lost once in the Eudorina-Pleodorina-Volvox lineage (Kirk 2006). Oogamy also evolved independently in almost all the lineages of eukaryotes that evolved multicellularity, e.g., in other Chlorophyta (green algae), Embryophyta (land plants), Rhodophyta (red algae), Phaeophyceae (brown algae), fungi, and animals (Kirk 2006). However, except for one lineage, there are no molecular genetic data relating the sex-determining loci of oogamous organisms to the mating types of their isogamous ancestors. The only lineage for which we have such data is the volvocine lineage.
Sexual development in V. carteri and C. reinhardtii is controlled by a large, multigenic, haploid sex-determining locus (mating-type locus) that is located on homologous chromosomes in the two species and that segregates as a single Mendelian trait in each. In C. reinhardtii, the mating-type locus is a 200- to 300-kb region within which the order of genes is rearranged between sexes (plus and minus mating type); thus, meiotic recombination is suppressed in this region (Ferris et al. 2002; Merchant et al. 2007). Relative to C. reinhardtii, the sex-determining locus of V. carteri has undergone a remarkable divergence in both sexes and has expanded fivefold (Kianianmomeni et al. 2008; Ferris et al. 2010; Prochnik et al. 2010).
Only the sex-specific genes MID (Ferris and Goodenough 1997) and MTD1 (Lin and Goodenough 2007) from C. reinhardtii have clear homologs in V. carteri, namely in the male mating-type locus (Nozaki et al. 2006; Ferris et al. 2010). The MID gene of C. reinhardtii was shown to be both necessary and sufficient to cause cells to differentiate as gametes of the minus mating type (Ferris and Goodenough 1997). Thus, the MID homologs indicate that maleness in the oogamous V. carteri might have evolved from the minus mating type of its isogamous unicellular ancestor (Nozaki et al. 2006; Nozaki 2008).
Further analysis of the mating-type locus of V. carteri identified several male- and female-specific genes without detectable homologs in C. reinhardtii (Ferris et al. 2010), but their functions remain to be investigated.
A key protein in sexual development seems to be the gender-specific retinoblastoma-related protein RBR1 (Kianianmomeni et al. 2008; Hallmann 2009a). The RBR1 gene maps to the sex-determining locus and exhibits divergent evolution in females and males; in addition, several splice variants of the RBR1 transcript exist (Kianianmomeni et al. 2008). An RBR1-related homolog exists not only in C. reinhardtii, where it is called MAT3 (Gillham et al. 1987; Armbrust et al. 1995; Umen and Goodenough 2001), but also in almost all eukaryotes (Hallmann 2009b), which highlights the importance of RBR1.
The information about the function of the genes described above is promising, but clearly more work is required to understand the evolution and functionality of the male–female dichotomy at a molecular level.
Conclusions and prospects
The gradual evolutionary transition in volvocine algae from unicellular species to multicellular species with a full division of labor between germline and somatic cells and with male–female dichotomy not only exemplifies the evolution of cellular cooperation from cellular autonomy but is also a prime example of Darwin’s notion that complex traits arise via evolution by small steps (Darwin 1872). However, the observed disappearance of innovations in the evolution of volvocine algae also indicates that evolution is not a one-way street from simple to complex organisms.
On the basis of the above-mentioned traits characteristic of volvocine algae, and taking into account previous considerations by Hoops (1997), Herron and Michod (2008) and Kirk (2005), the transition to differentiated multicellularity in asexual volvocine algae encompasses the following nine developments:
-
1.
an increase in the number of cleavage divisions and an incomplete cytokinesis that generates a cytoplasmic bridge network between cells;
-
2.
inversion of the (spheroidal) cell sheet to correct a maladaptive arrangement produced during cleavage; this inversion is the solution to a special problem found only in volvocine embryos;
-
3.
development and expansion of a multifunctional ECM, which displays a dynamic and multifunctional interface; the ECM holds cells in the appropriate places on the surface of the spheroid, and developmentally controlled ECM-specific enzymes allow for ECM remodeling during ontogenesis;
-
4.
modification of the breaststroke-type swimming motion of unicellular species to a beating mode in which the effective strokes of both flagella of each cell beat in the same direction, which is accomplished by rotation of the basal bodies;
-
5.
modification of flagellar beating direction in such a way that it causes rotation of the spheroid, which is required for phototaxis, rather than rotation of the cell;
-
6.
invention of organismal polarity with gradients in cell size, cell-to-cell distance, eyespot size, and light sensitivity along the anterior–posterior axis;
-
7.
invention of partial germ–soma division of labor; all cells first differentiate into motile biflagellate (somatic-like) cells and then later at least some cells redifferentiate and turn into nonmotile gonidia, while all the other cells remain as motile biflagellate cells and eventually die;
-
8.
evolution of full germ–soma division of labor between somatic and germline cells (germ–soma dichotomy); gonidia differentiate without first developing functional flagella and contributing to the motility of the organism; all the other cells develop as sterile somatic cells and eventually die;
-
9.
evolution of asymmetric cell division producing large and small daughter cells and bifurcation of the cell division program.
The stepwise transition to multicellularity with differentiated cell types in volvocine algae likely has parallels in the transitions to multicellularity that occurred in other eukaryotic lineages (Fig. 2). The frequent and convergent evolution of differentiated multicellularity seems to indicate that it can be achieved relatively easily. This speculation is corroborated by a recent mathematical model, which shows that complete germ–soma differentiation can be achieved easily and quickly (within a million generations) via the evolution of developmental plasticity (Gavrilets 2010).
Volvocine algae that exhibit a partial or complete division of labor in the asexual cycle exhibit a similar division of labor in the sexual cycle. Thus, for example, in V. carteri asexual gonidia, eggs and sperm-forming cells are all set apart from somatic cells by asymmetric cleavage divisions, whereas Volvox species that form gonidia without benefit of asymmetric division form prospective eggs and sperm-forming cells by similar mechanisms. Sex seems to provide the following advantages for volvocine algae (Maynard Smith 1978; Dawes 1981; Coleman 1983; Bernstein et al. 1985; Kirk and Kirk 1986; Goodenough et al. 1995; Kirk 1998; Burt 2000; Cavalier-Smith 2002; Colegrave et al. 2002; Colegrave 2002; Kaltz and Bell 2002; Hallmann 2003b):
-
a.
survival during life-threatening conditions by the production of stress-resistant zygotes (zygospores);
-
b.
creation of genetic variation, which is required for evolution;
-
c.
recombinational DNA repair during meiosis because homologous chromosomes pair at that time.
Like differentiated multicellularity in asexual reproduction, different cell types in sexual males and females with male–female dichotomy were probably also achieved relatively easily in evolution. In the first phase of the sexual life cycle of V. carteri females, only the first asymmetrical cell division had to be postponed from the sixth to the seventh division cycle. The large reproductive cell initials develop into haploid eggs, which resemble haploid gonidia, albeit somewhat smaller; in fact, after a waiting period, unfertilized eggs can develop into gonidia and enter the asexual cycle. Likewise, in the first phase of the sexual life cycle of V. carteri males, only the asymmetrical cell division had to be postponed from the sixth to the eighth division cycle. However, these large reproductive cell initials later undergo six additional symmetric division cycles to form small haploid biflagellate sperm cells. Such traits and cleavage programs related to fertilization and reproduction are known to evolve extremely rapidly (Rice 1998; Palumbi 1998; Howard et al. 1998; Gavrilets 2000). After fertilization, the formation of dormant, heavily walled, diploid zygotes/zygospores in multicellular species is quite similar to that observed in unicellular species.
Taken together, the evolution of multicellularity, development of sterile somatic cells, and generation of a male–female dichotomy are clearly among life’s greatest innovations. However, we learn from volvocine algae that the transition to such complex life is probably much easier to achieve than might be commonly believed. Volvox and its relatives seem to be ideal model organisms for addressing fundamental issues in the evolution of organismic complexity and for discovering universal rules that characterize this transition.
The post-genomic era is just beginning for Chlamydomonas and Volvox. Future comparative genomics studies of gene expression and gene regulation in volvocine algae of different organismic complexities promise further insights into how the ancestral genes of unicellular species have been changed and co-opted to play modified roles and allow for the development of colonial and multicellular descendants.
References
Armbrust EV, Ibrahim A, Goodenough UW (1995) A mating type-linked mutation that disrupts the uniparental inheritance of chloroplast DNA also disrupts cell-size control in Chlamydomonas. Mol Biol Cell 6:1807–1818
Baldauf SL (2003) The deep roots of eukaryotes. Science 300:1703–1706
Bell G (1985) The origin and early evolution of germ cells as illustrated by the Volvocales. In: Halvorson HO, Monroy A (eds) The origin and evolution of sex. Alan R. Liss, New York, pp 221–256
Bell G (1998) Model metaorganism. Science 282:248
Bernstein H, Byerly HC, Hopf FA, Michod RE (1985) Genetic damage, mutation, and the evolution of sex. Science 229:1277–1281
Blankenship JE, Kindle KL (1992) Expression of chimeric genes by the light-regulated cabII-1 promoter in Chlamydomonas reinhardtii: a cabII-1/nit1 gene functions as a dominant selectable marker in a nit1 − nit2 − strain. Mol Cell Biol 12:5268–5279
Bonner JT (1965) Size and cycle. An essay on the structure of biology. Princeton University Press, Princeton
Bonner JT (1974) On development. The biology of form. Harvard University Press, Cambridge
Bonner JT (1988) The evolution of complexity by means of natural selection. Princeton University Press, Princeton
Bonner JT (1993) Life cycles. Reflections of an evolutionary biologist. Princeton University Press, Princeton
Bonner JT (1998) The origins of multicellularity. Integr Biol 1:27–36
Bonner JT (2000) First signals: the evolution of multicellular development. Princeton University Press, Princeton
Bonner JT (2003) On the origin of differentiation. J Biosci 28:523–528
Burt A (2000) Perspective: sex, recombination, and the efficacy of selection—was Weismann right? Evolution 54:337–351
Buss LW (1987) The evolution of individuality. Princeton University Press, Princeton
Carroll SB (2001) Chance and necessity: the evolution of morphological complexity and diversity. Nature 409:1102–1109
Cavalier-Smith T (1991) Cell diversification in heterotrophic flagellates. In: Patterson DJ, Larsen J (eds) The biology of free-living heterotrophic flagellates. Clarendon Press, Oxford, pp 113–131
Cavalier-Smith T (2002) Origins of the machinery of recombination and sex. Heredity 88:125–141
Cheng Q, Fowler R, Tam LW, Edwards L, Miller SM (2003) The role of GlsA in the evolution of asymmetric cell division in the green alga Volvox carteri. Dev Genes Evol 213:328–335
Cheng Q, Pappas V, Hallmann A, Miller SM (2005) Hsp70A and GlsA interact as partner chaperones to regulate asymmetric division in Volvox. Dev Biol 286:537–548
Colegrave N (2002) Sex releases the speed limit on evolution. Nature 420:664–666
Colegrave N, Kaltz O, Bell G (2002) The ecology and genetics of fitness in Chlamydomonas. VIII. The dynamics of adaptation to novel environments after a single episode of sex. Evolution 56:14–21
Coleman AW (1983) The roles of resting spores and akinetes in chlorophyte survival. In: Fryxell GA (ed) Survival strategies of the algae. Cambridge University Press, Cambridge, pp 1–21
Coleman AW (1999) Phylogenetic analysis of “Volvocaceae” for comparative genetic studies. Proc Natl Acad Sci USA 96:13892–13897
Darwin CR (1872) The origin of species by means of natural selection, or the preservation of favoured races in the struggle for life, 6th edn. John Murray, London
Dawes IW (1981) Sporulation in evolution. In: Carlile MJ, Collins JF, Moseby BEB (eds) Molecular and cellular aspects of microbial evolution. Cambridge University Press, New York, pp 85–130
Debuchy R, Purton S, Rochaix JD (1989) The argininosuccinate lyase gene of Chlamydomonas reinhardtii: an important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J 8:2803–2809
Desnitski AG (1993) On the origins and early evolution of multicellularity. Biosystems 29:129–132
Desnitski AG (1995) A review on the evolution of development in Volvox: morphological and physiological aspects. Eur J Protistol 31:241–247
Duncan L, Nishii I, Howard A, Kirk D, Miller SM (2006) Orthologs and paralogs of regA, a master cell-type regulatory gene in Volvox carteri. Curr Genet 50:61–72
Duncan L, Nishii I, Harryman A, Buckley S, Howard A, Friedman NR, Miller SM (2007) The VARL gene family and the evolutionary origins of the master cell-type regulatory gene, regA, in Volvox carteri. J Mol Evol 65:1–11
Fernández E, Schnell R, Ranum LP, Hussey SC, Silflow CD, Lefebvre PA (1989) Isolation and characterization of the nitrate reductase structural gene of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 86:6449–6453
Ferris PJ, Goodenough UW (1997) Mating type in Chlamydomonas is specified by mid, the minus-dominance gene. Genetics 146:859–869
Ferris PJ, Armbrust EV, Goodenough UW (2002) Genetic structure of the mating-type locus of Chlamydomonas reinhardtii. Genetics 160:181–200
Ferris P, Olson BJ, De Hoff PL, Douglass S, Casero D, Prochnik S, Geng S, Rai R, Grimwood J, Schmutz J, Nishii I, Hamaji T, Nozaki H, Pellegrini M, Umen JG (2010) Evolution of an expanded sex-determining locus in Volvox. Science 328:351–354
Gavrilets S (2000) Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403:886–889
Gavrilets S (2010) Rapid transition towards the division of labor via evolution of developmental plasticity. PLoS Comput Biol 6:e1000805
Gerisch G (1959) Die Zelldifferenzierung bei Pleodorina californica Shaw und die Organisation der Phytomonadinenkolonien. Arch Protistenkd 104:292–358
Gilles R, Gilles C, Jaenicke L (1984) Pheromone-binding and matrix-mediated events in sexual induction of Volvox carteri. Z Naturforsch C 39:584–592
Gillham NW, Boynton JE, Johnson AM, Burkhart BD (1987) Mating type linked mutations which disrupt the uniparental transmission of chloroplast genes in Chlamydomonas. Genetics 115:677–684
Goldschmidt-Clermont M (1991) Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker of site-directed transformation of Chlamydomonas. Nucleic Acids Res 19:4083–4089
Goodenough UW, Armbrust EV, Campbell AM, Ferris PJ (1995) Molecular genetics of sexuality in Chlamydomonas. Annu Rev Plant Physiol Plant Mol Biol 46:21–44
Greuel BT, Floyd GL (1985) Development of the flagellar apparatus and flagellar orientation in the colonial green alga Gonium pectorale (Volvocales). J Phycol 21:358–371
Grosberg RK, Strathmann R (2007) The evolution of multicellularity: a minor major transition? Annu Rev Ecol Evol Syst 38:621–654
Gruber H, Goetinck SD, Kirk DL, Schmitt R (1992) The nitrate reductase-encoding gene of Volvox carteri: map location, sequence and induction kinetics. Gene 120:75–83
Hallmann A (2002) The key function of the extracellular matrix in the evolution of multicellularity. Bioforum Int 6:315–317
Hallmann A (2003a) Experienced developers of multicellularity—the Volvocales. Bioforum Eur 6:326–328
Hallmann A (2003b) Extracellular matrix and sex-inducing pheromone in Volvox. Int Rev Cytol 227:131–182
Hallmann A (2006a) Morphogenesis in the family Volvocaceae: different tactics for turning an embryo right-side out. Protist 157:445–461
Hallmann A (2006b) The pherophorins: common, versatile building blocks in the evolution of extracellular matrix architecture in Volvocales. Plant J 45:292–307
Hallmann A (2009a) Key elements of the retinoblastoma tumor suppressor pathway in Volvox carteri. Commun Integr Biol 2:396–399
Hallmann A (2009b) Retinoblastoma-related proteins in lower eukaryotes. Commun Integr Biol 2:538–544
Hallmann A, Rappel A (1999) Genetic engineering of the multicellular green alga Volvox: a modified and multiplied bacterial antibiotic resistance gene as a dominant selectable marker. Plant J 17:99–109
Hallmann A, Sumper M (1994) Reporter genes and highly regulated promoters as tools for transformation experiments in Volvox carteri. Proc Natl Acad Sci USA 91:11562–11566
Hallmann A, Sumper M (1996) The Chlorella hexose/H+ symporter is a useful selectable marker and biochemical reagent when expressed in Volvox. Proc Natl Acad Sci USA 93:669–673
Hallmann A, Rappel A, Sumper M (1997) Gene replacement by homologous recombination in the multicellular green alga Volvox carteri. Proc Natl Acad Sci USA 94:7469–7474
Hallmann A, Godl K, Wenzl S, Sumper M (1998) The highly efficient sex-inducing pheromone system of Volvox. Trends Microbiol 6:185–189
Harris EH, Stern DB, Witman GB (2009) The Chlamydomonas sourcebook, 2nd edn. Academic Press, San Diego
Herron MD, Michod RE (2008) Evolution of complexity in the volvocine algae: transitions in individuality through Darwin’s eye. Evolution 62:436–451
Herron MD, Hackett JD, Aylward FO, Michod RE (2009) Triassic origin and early radiation of multicellular volvocine algae. Proc Natl Acad Sci USA 106:3254–3258
Herron MD, Desnitskiy AG, Michod RE (2010) Evolution of developmental programs in Volvox (Chlorophyta). J Phycol 46:316–324
Hoops HJ (1993) Flagellar, cellular and organismal polarity in Volvox carteri. J Cell Sci 104:105–117
Hoops HJ (1997) Motility in the colonial and multicellular Volvocales: structure, function, and evolution. Protoplasma 199:99–112
Hoops HJ, Floyd GL (1983) Ultrastructure and development of the flagellar apparatus and flagellar motion in the colonial green alga Astrephomene gubernaculifera. J Cell Sci 63:21–41
Howard DJ, Reece M, Gregory PG, Chu J, Cain ML (1998) The evolution of barriers to fertilization between closely related organisms. In: Howard DJ, Berlocher SH (eds) Endless forms: species and speciation. Oxford University Press, New York, pp 279–288
Kaiser D (2001) Building a multicellular organism. Annu Rev Genet 35:103–123
Kaltz O, Bell G (2002) The ecology and genetics of fitness in Chlamydomonas. XII. Repeated sexual episodes increase rates of adaptation to novel environments. Evolution 56:1743–1753
Kianianmomeni A, Nematollahi G, Hallmann A (2008) A gender-specific retinoblastoma-related protein in Volvox carteri implies a role for the retinoblastoma protein family in sexual development. Plant Cell 20:2399–2419
Kindle KL, Schnell RA, Fernandez E, Lefebvre PA (1989) Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J Cell Biol 109:2589–2601
Kindle KL, Richards KL, Stern DB (1991) Engineering the chloroplast genome: techniques and capabilities for chloroplast transformation in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 88:1721–1725
King N (2004) The unicellular ancestry of animal development. Dev Cell 7:313–325
Kirk DL (1988) The ontogeny and phylogeny of cellular differentiation in Volvox. Trends Genet 4:32–36
Kirk DL (1994) Germ cell specification in Volvox carteri. In: Marsh J, Goode J (eds) Germline development (Ciba Foundation Symposium 182). Wiley, Chichester, pp 2–30
Kirk DL (1995) Asymmetric division, cell size and germ-soma specification in Volvox. Sem Dev Biol 6:369–379
Kirk DL (1997) The genetic program for germ-soma differentiation in Volvox. Annu Rev Genet 31:359–380
Kirk DL (1998) Volvox: molecular-genetic origins of multicellularity and cellular differentiation. Developmental and cell biology series. Cambridge University Press, Cambridge
Kirk DL (1999) Evolution of multicellularity in the volvocine algae. Curr Opin Plant Biol 2:496–501
Kirk DL (2000) Volvox as a model system for studying the ontogeny and phylogeny of multicellularity and cellular differentiation. J Plant Growth Regul 19:265–274
Kirk DL (2001) Germ-soma differentiation in Volvox. Dev Biol 238:213–223
Kirk DL (2003) Seeking the ultimate and proximate causes of Volvox multicellularity and cellular differentiation. Integr Comp Biol 43:247–253
Kirk DL (2005) A twelve-step program for evolving multicellularity and a division of labor. BioEssays 27:299–310
Kirk DL (2006) Oogamy: inventing the sexes. Curr Biol 16:R1028–R1030
Kirk DL, Kirk MM (1986) Heat shock elicits production of sexual inducer in Volvox. Science 231:51–54
Kirk MM, Kirk DL (2004) Exploring germ-soma differentiation in Volvox. J Biosci 29:143–152
Kirk DL, Nishii I (2001) Volvox carteri as a model for studying the genetic and cytological control of morphogenesis. Dev Growth Differ 43:621–631
Kirk DL, Viamontes GI, Green KJ, Bryant JL Jr (1982) Integrated morphogenetic behavior of cell sheets: Volvox as a model. In: Subtelny S, Green PB (eds) Developmental order: its origin and regulation. Alan R. Liss, New York, pp 247–274
Kirk DL, Birchem R, King N (1986) The extracellular matrix of Volvox: a comparative study and proposed system of nomenclature. J Cell Sci 80:207–231
Kirk DL, Kirk MM, Stamer KA, Larson A (1990) The genetic basis for the evolution of multicellularity and cellular differentiation in the volvocine green algae. In: Dudley EC (ed) The unity of evolutionary biology. Dioscorides Press, Portland, pp 568–581
Kirk MM, Ransick A, McRae SE, Kirk DL (1993) The relationship between cell size and cell fate in Volvox carteri. J Cell Biol 123:191–208
Kirk MM, Stark K, Miller SM, Müller W, Taillon BE, Gruber H, Schmitt R, Kirk DL (1999) regA, a Volvox gene that plays a central role in germ-soma differentiation, encodes a novel regulatory protein. Development 126:639–647
Knoll AH (2003) Life on a young planet. Princeton University Press, Princeton
Knoll AH, Javaux EJ, Hewitt D, Cohen P (2006) Eukaryotic organisms in Proterozoic oceans. Philos Trans R Soc Lond B Biol Sci 361:1023–1038
Koufopanou V (1994) The evolution of soma in the Volvocales. Am Nat 143:907–931
Koufopanou V, Bell G (1991) Developmental mutants of Volvox: does mutation recreate the patterns of phylogenetic diversity? Evolution 45:1806–1822
Koufopanou V, Bell G (1993) Soma and germ: an experimental approach using Volvox. Proc R Soc Lond B Biol Sci 254:107–113
Larson A, Kirk MM, Kirk DL (1992) Molecular phylogeny of the volvocine flagellates. Mol Biol Evol 9:85–105
Lerche K, Hallmann A (2009) Stable nuclear transformation of Gonium pectorale. BMC Biotechnol 9:64
Lin H, Goodenough UW (2007) Gametogenesis in the Chlamydomonas reinhardtii minus mating type is controlled by two genes, MID and MTD1. Genetics 176:913–925
Mages H-W, Tschochner H, Sumper M (1988) The sexual inducer of Volvox carteri. Primary structure deduced from cDNA sequence. FEBS Lett 234:407–410
Mayfield SP, Kindle KL (1990) Stable nuclear transformation of Chlamydomonas reinhardtii by using a C. reinhardtii gene as the selectable marker. Proc Natl Acad Sci USA 87:2087–2091
Maynard Smith J (1978) The evolution of sex. Cambridge University Press, Cambridge
Maynard Smith J, Szathmáry E (1995) The major transitions in evolution. Freeman, Oxford
Medina M, Collins AG, Taylor JW, Valentine JW, Lipps JH, Amaral-Zettler L (2003) Phylogeny of Opisthokonta and the evolution of multicellularity and complexity in Fungi and Metazoa. Int J Astrobiol 2:203–211
Meissner M, Stark K, Cresnar B, Kirk DL, Schmitt R (1999) Volvox germline-specific genes that are putative targets of RegA repression encode chloroplast proteins. Curr Genet 36:363–370
Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Marechal-Drouard L, Marshall WF, Qu LH, Nelson DR, Sanderfoot AA, Spalding MH, Kapitonov VV, Ren Q, Ferris P, Lindquist E, Shapiro H, Lucas SM, Grimwood J, Schmutz J, Cardol P, Cerutti H, Chanfreau G, Chen CL, Cognat V, Croft MT, Dent R, Dutcher S, Fernandez E, Fukuzawa H, Gonzalez-Ballester D, Gonzalez-Halphen D, Hallmann A, Hanikenne M, Hippler M, Inwood W, Jabbari K, Kalanon M, Kuras R, Lefebvre PA, Lemaire SD, Lobanov AV, Lohr M, Manuell A, Meier I, Mets L, Mittag M, Mittelmeier T, Moroney JV, Moseley J, Napoli C, Nedelcu AM, Niyogi K, Novoselov SV, Paulsen IT, Pazour G, Purton S, Ral JP, Riano-Pachon DM, Riekhof W, Rymarquis L, Schroda M, Stern D, Umen J, Willows R, Wilson N, Zimmer SL, Allmer J, Balk J, Bisova K, Chen CJ, Elias M, Gendler K, Hauser C, Lamb MR, Ledford H, Long JC, Minagawa J, Page MD, Pan J, Pootakham W, Roje S, Rose A, Stahlberg E, Terauchi AM, Yang P, Ball S, Bowler C, Dieckmann CL, Gladyshev VN, Green P, Jorgensen R, Mayfield S, Mueller-Roeber B, Rajamani S, Sayre RT, Brokstein P, Dubchak I, Goodstein D, Hornick L, Huang YW, Jhaveri J, Luo Y, Martinez D, Ngau WC, Otillar B, Poliakov A, Porter A, Szajkowski L, Werner G, Zhou K, Grigoriev IV, Rokhsar DS, Grossman AR (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318:245–250
Michod RE (2007) Evolution of individuality during the transition from unicellular to multicellular life. Proc Natl Acad Sci USA 104(Suppl 1):8613–8618
Michod RE, Nedelcu AM, Roze D (2003) Cooperation and conflict in the evolution of individuality. IV. Conflict mediation and evolvability in Volvox carteri. Biosystems 69:95–114
Michod RE, Viossat Y, Solari CA, Hurand M, Nedelcu AM (2006) Life-history evolution and the origin of multicellularity. J Theor Biol 239:257–272
Miller SM (2002) Taming the fierce roller: an “enhanced” understanding of cellular differentiation in Volvox. BioEssays 24:3–7
Miller SM (2010) Volvox, Chlamydomonas, and the evolution of multicellularity. Nat Educ 3:65
Miller SM, Kirk DL (1999) glsA, a Volvox gene required for asymmetric division and germ cell specification, encodes a chaperone-like protein. Development 126:649–658
Miller DH, Mellman IS, Lamport DTA, Miller M (1974) The chemical composition of the cell wall of Chlamydomonas gymnogama and the concept of a plant cell wall protein. J Cell Biol 63:420–429
Minko I, Holloway SP, Nikaido S, Carter M, Odom OW, Johnson CH, Herrin DL (1999) Renilla luciferase as a vital reporter for chloroplast gene expression in Chlamydomonas. Mol Gen Genet 262:421–425
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Nakada T, Misawa K, Nozaki H (2008) Molecular systematics of Volvocales (Chlorophyceae, Chlorophyta) based on exhaustive 18S rRNA phylogenetic analyses. Mol Phylogenet Evol 48:281–291
Nedelcu AM (2009) Environmentally induced responses co-opted for reproductive altruism. Biol Lett 5:805–808
Nedelcu AM, Michod RE (2004) Evolvability, modularity, and individuality during the transition to multicellularity in volvocalean green algae. In: Schlosser G, Wagner GP (eds) Modularity in development and evolution. University of Chicago Press, Chicago
Nelson JA, Savereide PB, Lefebvre PA (1994) The CRY1 gene in Chlamydomonas reinhardtii: structure and use as a dominant selectable marker for nuclear transformation. Mol Cell Biol 14:4011–4019
Nozaki H (2003) Origin and evolution of the genera Pleodorina and Volvox (Volvocales). Biologia (Bratisl) 58:425–431
Nozaki H (2008) A new male-specific gene “OTOKOGI” in Pleodorina starrii (Volvocaceae, Chlorophyta) unveils the origin of male and female. Biologia (Bratisl) 63:772–777
Nozaki H, Itoh M, Sano R, Uchida H, Watanabe MM, Kuroiwa T (1995) Phylogenetic relationships within the colonial Volvocales (Chlorophyta) inferred from rbcL gene sequence data. J Phycol 31:970–979
Nozaki H, Ito M, Uchida H, Watanabe MM, Kuroiwa T (1997) Phylogenetic analysis of Eudorina species (Volvocaceae, Chlorophyta) based on rbcL gene sequences. J Phycol 33:859–863
Nozaki H, Ohta N, Takano H, Watanabe MM (1999) Reexamination of phylogenetic relationships within the colonial Volvocales (Chlorophyta): an analysis of atpB and rbcL gene sequences. J Phycol 35:104–112
Nozaki H, Misawa K, Kajita T, Kato M, Nohara S, Watanabe MM (2000) Origin and evolution of the colonial Volvocales (Chlorophyceae) as inferred from multiple, chloroplast gene sequences. Mol Phylogenet Evol 17:256–268
Nozaki H, Takahara M, Nakazawa A, Kita Y, Yamada T, Takano H, Kawano S, Kato M (2002) Evolution of rbcL group IA introns and intron open reading frames within the colonial Volvocales (Chlorophyceae). Mol Phylogenet Evol 23:326–338
Nozaki H, Mori T, Misumi O, Matsunaga S, Kuroiwa T (2006) Males evolved from the dominant isogametic mating type. Curr Biol 16:R1018–R1020
Palumbi SR (1998) formation and the evolution of gamete recognition loci. In: Howard DJ, Berlocher SH (eds) Endless forms: species and speciation. Oxford University Press, New York, pp 271–278
Pfeiffer T, Bonhoeffer S (2003) An evolutionary scenario for the transition to undifferentiated multicellularity. Proc Natl Acad Sci USA 100:1095–1098
Prochnik SE, Umen J, Nedelcu AM, Hallmann A, Miller SM, Nishii I, Ferris P, Kuo A, Mitros T, Fritz-Laylin LK, Hellsten U, Chapman J, Simakov O, Rensing SA, Terry A, Pangilinan J, Kapitonov V, Jurka J, Salamov A, Shapiro H, Schmutz J, Grimwood J, Lindquist E, Lucas S, Grigoriev IV, Schmitt R, Kirk D, Rokhsar DS (2010) Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science 329:223–226
Rausch H, Larsen N, Schmitt R (1989) Phylogenetic relationships of the green alga Volvox carteri deduced from small-subunit ribosomal RNA comparisons. J Mol Evol 29:255–265
Rice WR (1998) Intergenomic conflict, interlocus antagonistic coevolution, and the evolution of reproductive isolation. In: Howard DJ, Berlocher SH (eds) Endless forms: species and speciation. Oxford University Press, New York, pp 261–270
Rokas A (2008) The molecular origins of multicellular transitions. Curr Opin Genet Dev 18:472–478
Sachs JL (2008) Resolving the first steps to multicellularity. Trends Ecol Evol 23:245–248
Sager R, Granick S (1954) Nutritional control of sexuality in Chlamydomonas reinhardi. J Gen Physiol 37:729–742
Schiedlmeier B, Schmitt R, Müller W, Kirk MM, Gruber H, Mages W, Kirk DL (1994) Nuclear transformation of Volvox carteri. Proc Natl Acad Sci USA 91:5080–5084
Schmitt R (2001) Volvox carteri: molecular genetics of cell differentiation. Jpn J Protozool 34:7–12
Schmitt R (2003) Differentiation of germinal and somatic cells in Volvox carteri. Curr Opin Microbiol 6:608–613
Schnell RA, Lefebvre PA (1993) Isolation of the Chlamydomonas regulatory gene NIT2 by transposon tagging. Genetics 134:737–747
Schopf JW (1993) Microfossils of the early Archean Apex chert: new evidence of the antiquity of life. Science 260:640–646
Sleigh MA (1989) Protozoa and other protists. Edward Arnold, London
Sodeinde OA, Kindle KL (1993) Homologous recombination in the nuclear genome of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 90:9199–9203
Solari CA, Ganguly S, Kessler JO, Michod RE, Goldstein RE (2006a) Multicellularity and the functional interdependence of motility and molecular transport. Proc Natl Acad Sci USA 103:1353–1358
Solari CA, Kessler JO, Michod RE (2006b) A hydrodynamics approach to the evolution of multicellularity: flagellar motility and germ-soma differentiation in volvocalean green algae. Am Nat 167:537–554
Solari CA, Kessler JO, Goldstein RE (2007) Motility, mixing, and multicellularity. Genet Prog Evol Mach 8:115–129
Stark K, Schmitt R (2002) Genetic control of germ-soma differentiation in Volvox carteri. Protist 153:99–107
Starr RC (1969) Structure, reproduction and differentiation in Volvox carteri f. nagariensis Iyengar, strains HK 9 & 10. Arch Protistenkd 111:204–222
Starr RC (1970) Control of differentiation in Volvox. Dev Biol Suppl 4:59–100
Starr RC (1975) Meiosis in Volvox carteri f. nagariensis. Arch Protistenkd 117:187–191
Starr RC, Jaenicke L (1974) Purification and characterization of the hormone initiating sexual morphogenesis in Volvox carteri f. nagariensis Iyengar. Proc Natl Acad Sci USA 71:1050–1054
Stevens DR, Rochaix JD, Purton S (1996) The bacterial phleomycin resistance gene ble as a dominant selectable marker in Chlamydomonas. Mol Gen Genet 251:23–30
Sumper M, Hallmann A (1998) Biochemistry of the extracellular matrix of Volvox. Int Rev Cytol 180:51–85
Sumper M, Berg E, Wenzl S, Godl K (1993) How a sex pheromone might act at a concentration below 10–16 M. EMBO J 12:831–836
Szathmáry E, Smith JM (1995) The major evolutionary transitions. Nature 374:227–232
Tam LW, Lefebvre PA (1993) Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics 135:375–384
Tschochner H, Lottspeich F, Sumper M (1987) The sexual inducer of Volvox carteri: purification, chemical characterization and identification of its gene. EMBO J 6:2203–2207
Ueki N, Nishii I (2008) Idaten is a new cold-inducible transposon of Volvox carteri that can be used for tagging developmentally important genes. Genetics 180:1343–1353
Ueki N, Matsunaga S, Inouye I, Hallmann A (2010) How 5000 independent rowers coordinate their strokes in order to row into the sunlight: phototaxis in the multicellular green alga Volvox. BMC Biol 8:103
Umen JG, Goodenough UW (2001) Control of cell division by a retinoblastoma protein homolog in Chlamydomonas. Genes Dev 15:1652–1661
Weismann A (1889) The continuity of the germ plasm as a foundation of a theory of heredity. In: Poulton EB, Schonland S, Shipley AE (eds) Essays upon heredity and kinkred biological problems. Clarendon, Oxford
Weismann A (1892) Essays on heredity and kindred biological problems. Clarendon Press, Oxford
Weismann A (1893) The germ-plasm: a theory of heredity. Charles Scribner’s Sons, New York
Willensdorfer M (2008) Organism size promotes the evolution of specialized cells in multicellular digital organisms. J Evol Biol 21:104–110
Willensdorfer M (2009) On the evolution of differentiated multicellularity. Evolution 63:306–323
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Scott Russell.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Hallmann, A. Evolution of reproductive development in the volvocine algae. Sex Plant Reprod 24, 97–112 (2011). https://doi.org/10.1007/s00497-010-0158-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-010-0158-4