Abstract
Non-thermal plasma can be generated by gliding arc discharge units and is used in various applications including disinfection of the surface, inactivation of fungi, viruses, and bacteria. Degradation of the electrodes through prolonged use is common. It is caused by bombardment by plasma-charged particles causing changes to the structure and chemistry of its electrodes which in turn leads to a reduction in the efficiency of the plasma. In this study, the discharged plasma from a gliding arc unit was investigated using optical emission spectrometry (OES). Also studied were the electrodes of the unit after prolonged use. The structure and metallographic properties of the electrodes were evaluated using positron annihilation lifetime spectroscopy (PALS), X-ray diffraction analysis (XRD) and scanning electron microscopy—energy dispersive X-ray spectroscopy (SEM-EDX) and compared to data from new unused samples. The size of defects and holes were also compared using PALS. It was found that the intensity of species decreased significantly with prolonged use of the unit as the copper electrodes degraded. It was also found that the internal structure, as well as the surface of the used electrodes, had changed significantly compared to the unused samples, the radius of holes in the electrode material had decreased and the intensity of holes increased. Furthermore, the planes of the atomic structure of the copper electrode have moved closer. The approach developed in this study has been shown an effective non-destructive method for routine evaluation of the electrodes and maybe a development further for monitoring and quality assurance programs for maintaining high plasma efficiency as well as for research into improvements to electrode material design.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Matter in plasma form is chemically reactive with agents such as free radicals, excited species, and free electrons and is therefore used to increase the rate of chemical reactions [1–3]. Gliding arc (GA) discharge is a frequently used method for producing non-thermal plasma at atmospheric pressure [4]; applications have been in the production and enhancement of combustion [5], gas conversion [6], inactivation of fungi, viruses, and bacteria such as Covid-19 [7], processing and modification of surfaces [8] and de-contamination [9]. Spectral and electrical diagnostics of gliding arc depend on different parameters presented by Czernichowski and Nassar [10]. He measured the temperature of the vibrating and rotating molecular plasma gases and also the temperature and concentration of the free electrons. At quasi-equilibrium temperatures of 4000 to 6000 K and an unbalanced region with an electron temperature of about 10 000 K, they have reported vibration temperatures of about 3000 K, rotational temperatures of about 4300 K, and translation temperatures of about 1000 to 1500 K [11]. Progressive degradation of the electrodes which comes about as a result of bombardment by the plasma’s charged particles is an important consideration in the plasma’s efficient performance. In food contaminant removal applications, which is the application of interest in this study, the generation of reactive charged particles depends on the concentration of reactive oxygen species (ROS) and reactive nitrogen species (RNS) produced in the plasma. Various parameters such as the electrode material and temperature, plasma exposure time, discharge capacity, and carrier gas composition affect the type and concentration of these reactive species. To better evaluate the effect of electrode degradation, an understanding of the types and the basic mechanism of plasma formation and reactions is necessary. Plasma can be categorized as either existing in equilibrium or a state of non-equilibrium depending on the energy density, temperature, and electron density. Non-equilibrium plasma also referred to as cold plasma, has an imbalance of thermal kinetic energy of the electrons and ions and exists close to room temperatures and their chemical reactions involve mainly the generated electrons. As the electrons accelerate in the electric field between the cathode and anode electrodes, their energy is transferred to the gas molecules in their path through inelastic collisions and chemically excited species, free radicals, ions, etc. are formed [12]. Some researcher show that the plasma can change the lattice parameters due to changes in surface properties [13]. The effect of fusion plasma particles onto the structural materials is investigated in some researches. The displacements of interplanar spacing in copper is studied for α-particles and neutrons in [14]. The grain size effect on the radiation damage tolerance of cubic zirconia in low and high energy heavy ions is investigated by Radwan and El-Khabeary [15]. The erosion effect of neutral particles has been studied for the Experimental Advanced Superconducting Tokamak by Mu et al. [16].
Evaluation of the state of the copper electrodes and their surface defects has been carried out in the past using various techniques including intermediate X-ray scattering [10], pressure volume temperature (PVT) [17], 129Xe-nuclear magnetic resonance spectroscopy [18], and inverse gas chromatography (IGC) [19–22]. We propose positron annihilation lifetime spectroscopy (PALS) that has been used for the investigation of defect size in different metals [23].
PALS has been widely utilized in the measurement of the pores and the size of elementary free volume sites in solid material and is an established non-destructive method for detecting the porosity of materials, cavities, and defects of solids. PALS is currently one of the only practical and direct methods of determining the local free volume at the atomic scale and is used in a variety of materials such as metals, semiconductors, dielectrics, and polymers. In this spectroscopic method, the positron (an electron-sized particle but with a single positive charge) is used to penetrate the surface of the material [23–25].
Once inside matter, the particle rapidly slows down and thermalizes to the temperature of the surrounding material in a few pico-seconds via inelastic collisions and eventually annihilates with a free electron trapped in pores or free volume sites of the material. Just before annihilation, a bond forms between an electron and a positron often referred to as the positronium pair, Ps. Ps can be produced in one of two states: the triplet Ortho-Ps (O-Ps) state and the singlet Para-Ps (P-Ps) state that have parallel and anti-parallel spins, respectively. Eventually, the positron-electron pair annihilate usually releasing two near oppositely directed gamma rays with energies of 0.511 MeV [24].
The time-lapse between the emission of the positron from a radioactive material such as Na-22 and the detection of the annihilation gamma-rays is referred to as the lifetime of the positron; the statistical weight of O-Ps formation is almost three times that of P-Ps. Inside the material, the vacancies, voids, and defects, as well as the density of electrons at the location of the flaw affect the lifetime of the positron in the material. The pairing of the positron with an electron takes longer in large vacancies and the positron lifetime therefore increases. The characteristics of the ions, energy of the electrons, UV rays, and oxidants such as free radicals within the plasma can influence the size of the defects and therefore increase the positron lifetime [22–25].
In this paper, we report on the outcomes of our investigation into the degradation of copper rod electrodes using PALS, XRD, and SEM methods.
2 METHOD
The analyzed samples included new/unused and used/stroke/worn electrodes which had been in operation in the gliding plasma reactor for about 80 h. PALS was utilized for estimation of defect size. X-ray diffraction (XRD) was used for the identification of crystalline materials present in the electrodes and their characteristics, as well as the determination of unit cell dimensions. Scanning electron microscopy (SEM) was used for the identification of other chemical elements in the electrodes and the generation of magnified images for a complimentary analysis.
2.1 Measurement Set-Up for Gliding Arc Reactor
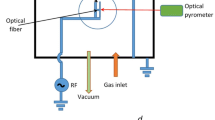
Figure 1 shows a schematic of the operation of the gliding arc reactor consisting of two or more metal electrodes. The application of a high voltage difference between the electrodes causes the creation of a low arc discharge [26, 27], which is characterized by a flame arc. The discharge is initiated in the space where the electrodes are closest and propagates along the surface of the electrodes at the rate of gas flow; the generation of plasma flame is governed by the discharge power and the rate of gas flow [28].
Figure 2 shows a schematic of the components of the gliding discharge reactor used in this study which included an AC power supply and an emission spectrum detection system. The reactor’s electrodes consisted of two copper rods with 3 mm diameters and lengths of 120 mm. The electrodes were inclined at 20 deg to each other and their closest separation was 2 mm apart. The potential difference between the electrodes was provided by a pure sine wave generator with voltages of about 4.48 kV at a frequency of 16.86 kHz.
Table 1 shows the characteristics of voltage and current sine waves. Detailed study of the process poses significant challenges due to the inherent instability of the generated electrical discharge, the short duration of the ignition process (nanoseconds), and masking of the process by the accompanying turbulent flow of the gas [28]. In this study, optical emission spectroscopy was used to analyze the gliding discharge plasma.
2.2 Measurement Setup for Positron Annihilation Lifetime Spectroscopy
Two opposing collinear plastic scintillation detectors (NT-1510) were used in conjunction with a conventional spectrometer to derive an indirect estimation of the volume of vacancies in the plasma gliding-arc discharges electrodes. The time resolution of the detection system was about 360 ps as measured for the detection of the coincidence gamma emissions from 60Co. The channel width was adjusted to 22 ps to provide the required spectroscopic resolution. To achieve the required statistical certainty of one million counts, i.e., about 0.1%, the acquisition time was about 72 h at a temperature of about 23°C for used and new electrodes shown in Fig. 3.
The new electrodes (each of 4 mm diameter and 100 mm in length) had shiny surfaces (Fig. 3, a) whilst the used electrodes had matt surfaces with a dark coating indicating the presence of a layer of oxidation products flowing about eight months of use (Fig. 3, b); the corrosion and pits arising from damage by the impact of plasma particles were readily visible on the electrode surfaces.
The shape of the ideal spectrum of the PALS equipment can be described by
where y(t) is the counting rate as a function of the time t, τi is the mean lifetime of the ith component, and Ii is the intensity of the ith component. The positron lifetime recorded for metal is analyzed by two components, τ1 and τ2. The experimental spectrum of PALS is analyzed by LT-10 software [24] for the intensities and lifetimes of the various components. It calculates the positron lifetime from the acquired spectra for each of the samples; the software decomposed the spectra into two exponential components of a lifetime, τi (ns) and intensity, Ii (%). The analyses were preceded by adjustment for the initial value of τi, background level, and the zero channel and a source-dependent correction factor needed to be applied. To evaluate the PALS system, a well-annealed pure Ni sample was analyzed and the positron lifetime measured was found to be about 110 ps; this was consistent with the reference values [20, 22].
The input of the analysis software was the spectrum obtained from the PALS device and allowed the calculation of the intensity and τ for each sample. The mean size of the holes was correlated to the longest-lived component of the lifetime spectrum. According to a quantum mechanical model, first introduced by Tao [14] for the O-Ps lifetime, many description for the properties of micro voids are presented. Values of micro voids or free volume have been calculated using the group contribution method or estimation of the occupied volume (van der Waal’s). The size of the free volume hole can be calculated using the semi-empirical equation, the holes can be considered as spherical and their radii (R) can be calculated [19]:
where τ is calculated using LT-10 software by fitting two exponential functions to the real data that was obtained from the PALS system, R denotes the mean radius of the defects, ΔR = 1.656 Å, and R can be obtained by Eq. (2).
3 RESULTS AND DISCUSSION
With use, the electrodes of the plasma gliding-arc degrade and the hole density in their body increase resulting in a fall in the efficiency of the unit, that is, the concentration of ROS and RNS produced in the plasma falls. As well as analysis of the plasma annihilation photons spectra using the optical emission spectrometry, OES method and measurement of the intensity of the chemical two species, new and used electrodes were examined for wear using metallographic examinations and evaluated using PALS. The structure of the electrodes was also investigated using XRD and SEM analysis. An X-ray diffractometer (STOE MP-Stady, Germany) which has a CuKα X-ray beam of wavelength 1.5406 Å, was used to examine microstructural changes due to irradiation. The diffractometer operated at 30 mA and 40 kV. The diffraction range profiles were 20°–120° with step size 0.02° in a continuous scan time of 1 s per step.
3.1 OES Plasma Spectra Comparison
Figures 4 and 5 show the results of the OES analysis for new and used electrodes, respectively; they indicate significant changes in the electrodes. The spectra show the formation of reactive species ROS and RNS species with different wavelengths for each sample. For both data sets, analysis in the range 20 to 550 nm, showed species including NO (at 226, 236, and 246 nm), OH (at 309 nm), N3 (at 316 and 337 nm), and O (at 357 nm). Comparing the two spectra showed that the intensity of the peaks (which could be correlated to the concentration of the species) declined significantly for the plasma generated by the used electrodes.
Decreased electric field and thus reduced plasma intensity can have several causes. For example, creating excessive holes in the electrode surface and corrosion due to high voltage electrical discharge can be one of the factors weakening the electric field [10–17]. Another reason is the formation of an insulating layer on the surface of these electrodes. These insulation layers are generally copper oxides. One of the reasons for the reduction of the electric field and consequently the reduction of the plasma intensity is the sputtering and the creation of a hole on the electrode surface.
It has been shown that in the presence of significant holes population arising from excessive wear within the body and surface of the electrodes, the electric field generating the plasm is suppressed leading to a corresponding fall in the unit’s efficiency [10–12]. The formation of an insulating layer (often copper oxide) on the surface of the electrodes can also lead to a reduction in the electric field as well as causing sputtering and the creation of a hole on the electrode surfaces.
3.2 PALS, XRD, and SEM Evaluation
The changes of electrodes were with energy dispersive X-ray spectroscopy (EDX) methods. Figures 6 and 7 show the measured spectra from PALS for the new and used electrodes respectively; also shown are the fitted curves to each spectrum. The residual differences between the data points (red dots) and the fitted theoretical exponentials (blue curve) are presented at the bottom of each figure. The spectra appear similar on the first inspection, however, on closer inspection, there are differences in the descending sections as indicated by the arrows.
The values of intensity and τ (see Eq. (2)) for the new and used electrodes were calculated from the fitted exponential curves using the LT10 software and are shown in Table 2; degree of fit, χ, was between 1 to 2. The results showed that τ2 decreased and I2 increased in the used electrode compared to the new electrode. Using Eq. (2), the average radius of the holes in the used electrode was found to be about 2.1 and about 1.45 Å in the new electrode.
The corresponding mean volume of the holes, Vh (Å3) = 4/3πR3 were also calculated and is shown in Table 2. The results indicate that the average radius of the holes in the used copper electrodes had been reduced by a statistically significant amount, i.e., about 68% leading to a corresponding increase in the number of the vacancies.
It is noticeable that a radioactive (β+) source has the exponential function for the stopping profile of energetic positrons as:
where ρ is the density of the solid and Emax is the maximum energy of the continuous β+ radiation spectrum. For 22Na with Emax = 0.54 MeV that is the most common isotope for positron experiments, the characteristic penetration depth 1/α is 384 μm in Cu. Therefore, positrons can probe the bulk of a Cu. Also, the size of the oxide particles is in the range 50 to 100 nm. According to Eq. (3), the range of positron penetration in Cu is in the range of micrometers. Therefore, in PALS method, we can have information of different layer of Cu such as oxidized layers and sub-oxidized layers [29].
Table 3 shows the results of XRD measurements between 20° to 110° for new and after used electrodes. The peaks of copper for new electrodes have corresponded well with the pure cubic copper phase (JCPDF no. 00-004-0836); XRD peaks are at 43.2170°, 50.3875°, 74.1105°, and 89.9195° and are indexed as (111), (200), (220), and (311) hkl planes of the Cu phase (Fig. 8). The results of the after used electrodes show that XRD peaks had a small relative displacement and were observed at 43.2364°, 50.3934°, 74.1170°, and 89.9683°. Table 3 shows that d-spacing at 89.9195° for the 311 plates were 1.09013 and 1.08967 Å for new and used electrodes, respectively, with corresponding X-ray intensities of 78.46 and 50.59%. The change of the d-spacing value was about 0.001 and slight atomic displacement occurred by the effect of residual stress. This displacement results in slightly change in the interlayer spacing, the lattice parameter and the crystallite size of Cu. Measurement of peak width (FWHM) also showed that the average crystallite size decreased after radiation. The decreased crystal size can be due to the increase in the vacancy, interstitial, substitutional defects in Cu. The trend is similar to the change of characters of Cu in O2- and N2-plasma [13, 30]. Therefore, the plates were closer to each other in the worn electrodes with a decrease in the mass of the electrode material, and bombardment of the plasma particles had altered the copper lattice and the holes were decreased by wear; these findings are in line with the PALS outcomes.
Figure 9 shows the SEM-EDX spectra for new and used electrodes. The elemental composition of the electrodes was determined from the two spectra and normalized to 100% of the CuKα peak. For the used electrodes additional elements were identified including also carbon (used for the coating of the electrode) and oxygen. Informing the SEM image, both carbon and oxygen (Cu : O ratio of 3 : 1) were removed from the dataset.
Figure 10 shows the resulting morphology of the electrodes’ surfaces with standard and higher magnification. The images reveal that bombardment by the plasma particles had caused significant wear to the surface of the used electrode.
4 CONCLUSIONS
The results of OES show that the intensity of the plasma-produced chemical species of interest had decreased significantly for the used electrodes and that the nature of the damage was the main cause of the fall. PALS, XRD, and SEM were shown to be effective complementary analysis methods for revealing the level of deep wear of the electrodes. PALS was used successfully for estimation of the number of the holes and their effective radii and were able to estimate the extent of electrode damage. The XRD method was shown to be capable of estimating the distance between the copper lattice and the corresponding results were found to be consistent with data from PALS analysis, i.e., both showed closer plate proximity in used electrodes and a net fall in the bulk mass of material. SEM images proved useful in the visualization of the extent and density of defects on electrode surfaces. It has been shown that the approach reported in this paper be used as an effective non-destructive method for routine evaluation of copper electrodes and maybe development for monitoring and quality assurance programs for maintaining high plasma efficiency as well as for research into improvements to electrode material design.
REFERENCES
Fridman, A., Nester, S., Kennedy, L.A., Saveliev, A., and Mutaf-Yardimci, O., Gliding arc gas discharge, Prog. Energ. Combust., 1999, vol. 25, no. 2, pp. 211–231. https://doi.org/10.1016/S0360-1285(98)00021-5
Gharagozalian, M., Dorranian, D., and Ghoranneviss, M., Water treatment by the AC gliding arc air plasma, J. Theor. Appl. Phys., 2017, vol. 11, pp. 171–180. https://doi.org/10.1007/s40094-017-0254-z
Solyakov, D.G., Volkova, Yu.Ye., Ladygina, M.S., Merenkova, T.M., Marchenko, A.K., Garkusha, I.E., Petrov, Yu.V., Chebotarev, V.V., Makhlai, V.A., Kulik, M.V., Staltsov, V.V., and Yeliseyev, D.V., Distributions of magnetic field and current in pinching plasma flows: effect of axial magnetic field, Eur. Phys. J. Plus, 2021, vol. 136, p. 566, https://doi.org/10.1140/epjp/s13360-021-01170-z
Simon, A., Anghel, S.D., Papiu, M., and Dinu, O., Physical and analytical characteristics of an atmospheric pressure argon–helium radiofrequency capacitively coupled plasma, Spectrochim. Acta, Part B, 2010, vol. 65, no. 4, pp. 272–278. https://doi.org/10.1016/j.sab.2010.02.002
Kusano, Y., Sorensen, B.F., Andersen, T.L., Toftegaard, H.L., Leipold, F., Salewski, M., Sun, Z.W., Zhu, J.J., Li, Z.S., and Aldén, M., Water-cooled non-thermal gliding arc for adhesion improvement of glass-fibre-reinforced polyester, J. Phys. D. Appl. Phys., 2013, vol. 46, no. 13, p. 135203. https://doi.org/10.1088/0022-3727/46/13/135203
Bo, Z., Wu, E., Yan, J., Chi, Y., and Cen, K., Gliding arc discharges with phase-chopped voltage supply for enhancement of energy efficiency in volatile organic compound decomposition, Rev. Sci. Instrum., 2013, vol. 84, no. 1, p. 016105. https://doi.org/10.1063/1.4775720
Mohamed, H., Nayak, G., Rendine, N., Wigdahl, B., Krebs, F.C., Bruggeman, P.J., and Miller. V., Non-thermal plasma as a novel strategy for treating or preventing viral infection and associated disease, Front. Phys., 2021, vol. 9, p. 683118. https://doi.org/10.3389/fphy.2021.683118
Ju, Y.G. and Sun, W.T., Plasma assisted combustion: Dynamics and chemistry, Prog. Energ. Combust., 2015, vol. 48, pp. 21–83. https://doi.org/10.1016/j.pecs./j.proci.2014.05.073
Yin, Z., Eckert, Z., Adamovich, I.V., and Lempert, W.R., Time-resolved radical species and temperature distributions in an Ar–O2–H2 mixture excited by a nanosecond pulse discharge, Proc. Combust. Inst., 2015, vol. 35, no. 3, pp. 3455–3462. https://doi.org/10.1364/OE.25.020243
Czernichowski, A., Nassar, H., Ranaivosoloarimanana, A., Fridman, A.A., Simek, M., Musiol, K., and Dittrichova, L., Spectral and electrical diagnostic of gliding arc, Acta Phys. Pol. A, 1996, vol. 89, no. 5, pp. 595–604. https://doi.org/10.12693/APhysPolA.89.595
Zhu J., Gao, J., Ehn, A., Adlen, M., Li, Zh., Moseev, D., Kusano, Y., Salewski, M., Alpers, A., Gritzmann, P., and Schwenk, M., Measurements of 3D slip velocities and plasma column lengths of a gliding arc discharge, Appl. Phys. Lett., 2015, vol. 106, no. 4, p. 044101. https://doi.org/10.1063/1.4906928
Song, H.H. and Ryong, J.R., Structural change accompanying volume change in amorphous polystyrene as studied by small and intermediate angle X-ray scattering, Macromolecules, 1987, vol. 20, no. 11, pp. 2723–2732. https://doi.org/10.1021/ma00177a016
Jang, H.J., Jung, E.Y., Parsons, T., Tae, H.-S. and Park, Ch.-S., A review of plasma synthesis methods for polymer films and nanoparticles under atmospheric pressure conditions, Polymers, 2021, vol. 13, p. 2267. https://doi.org/10.3390/polym13142267
Kalita, P., Ghosh, S., Gutierrez, G., Rajput, P., Grover, V., Sattonnay, G., and Avasthi, D.K., Grain size effect on the radiation damage tolerance of cubic zirconia against simultaneous low and high energy heavy ions: Nano triumphs bulk, Sci. Rep., 2021, vol. 11, p.10886. https://doi.org/10.1038/s41598-021-90214-6
Radwan, S. and El-Khabeary, H., Influence of high-energy particles on copper and stainless steel in fusion reactor materials, Can. J. Phys., 2021, vol. 99, no. 11, pp. 954–968. https://doi.org/10.1139/cjp-2020-0566
Mu, L., Ding, R., Zhu, Y.B., Chen, J.L., and Li, S., Development of a time-of-flight low-energy neutral particle analyzer for EAST tokamak, Rev. Sci. Instrum., 2018, vol. 89, no. 10, p. 10I117. https://doi.org/10.1063/1.5038936
Abiev, R.Sh., Sladkovskiy, D.A., Semikin, K.V., Murzin, D.Yu., and Rebrov, E.V., Non-thermal plasma for process and energy intensification in dry reforming of methane, Catalysts, 2020, vol. 10, no. 11, p. 1358. https://doi.org/10.3390/catal10111358
Sahin-Dinc, F., Yahsi, U., and Sedlacek, T., Interrelationships of pressure-dependent hole fraction and elongational viscosity in polymer melts, Adv. Polym. Technol., 2019, p. 9493769. https://doi.org/10.1155/2019/9493769
Pethrick, R.A., Positron annihilation—a probe for nanoscale voids and free volume?, Prog. Polym. Sci., 1997, vol. 22, no. 1, pp. 1–47. https://doi.org/10.1016/S0079-6700(96)00023-8
Golemme, G., Nagy, J.B., Fonseca, A., Algieri, C., and Yampolskii, Yu., 129Xe-NMR study of free volume in amorphous per-fluorinated polymers: comparison with other methods, Polymer, 2003, vol. 44, no. 17, pp. 5039–5045. https://doi.org/10.1016/S0032-3861(03)00450-6
Yampolskii, Y. and Belov, N., Investigation of polymers by inverse gas chromatography, Macromolecules, 2015, vol. 48, no. 19, pp. 6751–6767. https://doi.org/10.1021/acs.macromol.5b00895
Li, J., Xiong, B., Yin, Ch., Zhang, Xi., Zhou, Y., Wang, Zh., Fang P., and He, Ch., Free volume characteristics on water permeation and salt rejection of polyamide reverse osmosis membranes investigated by a pulsed slow positron beam, J. Mater. Sci., 2018, vol. 53, no. 23, pp. 16132–16145. https://doi.org/10.1007/s1085
Positrons in Solids, Hautojärvi, P., Ed., Berlin: Springer, 1979. https://doi.org/10.1007/978-3-642-81316-0
Biganeh, A., Kakuee, O., Rafi-Kheiri, H., Lamehi-Rachti, M., Sheikh, N., and Yahaghi, E., Positron annihilation lifetime and doppler broadening spectroscopy of polymers, Radiat. Phys. Chem., 2020, vol. 166, p. 108461. https://doi.org/10.1016/j.radphyschem.2019.108461
Kansy, J., Microcomputer program for analysis of positron annihilation lifetime spectra, Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip., 1996, vol. 374, no. 2, pp. 235–244. https://doi.org/10.1016/0168-9002(96)00075-7
Bourennane, H., Gueribiz, D., and Fréour, S., Modeling the effect of damage on diffusive behavior in a polymeric matrix composite material, J. Reinf. Plast. Compos., 2019, vol. 38, no. 15, pp. 717–733. https://doi.org/10.1177/0731684419845479
Fridman, A., Plasma Chemistry, New York: Cambridge Univ. Press, 2008. https://doi.org/10.1017/CBO9780511546075
Gnapowski, S., Kalinowska-Ozgowicz, E., Sniadkowski, M., and Pietraszek, A., Metallographic comparison of copper and brass plasma reactor electrodes after a month of operation, Preprints, 2019, pp. 1–11. https://doi.org/10.20944/preprints201907.0109.v1
Tuomisto, F. and Makkonen, I., Defect identification in semiconductors with positron annihilation: Experiment and theory, Rev. Mod. Phys., 2013, vol. 85, no. 4, pp. 1583–1631. https://doi.org/10.1103/RevModPhys.85.1583
Chiang, Ch.-Ch., Chen, M.-Ch., Li, L.J., Wu, Zh.-Ch., Jang, S.-M., and Liang, M.-S., Effects of O2 and N2 plasma treatments on copper surface, Jpn. J. Appl. Phys., 2004, vol. 43, no. 11A, pp. 7415–7418.
ACKNOWLEDGMENTS
The authors would like to thank the Nuclear Laboratory of Imam Khomeini International University and Plasma and Fusion Research School, Nuclear Science and Technology Research Institute (NSTRI) for their cooperation in the preparing of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ramezani, M.B., Yahaghi, E. & Nohekhan, M. An Empirical Study of Electrode Degradation in Gliding Arc Discharge. Russ J Nondestruct Test 58, 632–642 (2022). https://doi.org/10.1134/S106183092207004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106183092207004X