Abstract
The opinion exists that water stability is provided by hydrophobic bonds between organic soil particles; however, there are works in which the main role in the occurrence of this property is assigned to the presence of hydrophilic organic substances in soils. The goal of this study is to clarify the nature of the bonds (hydrophilic or hydrophobic) that ensure the water stability of soils. We used samples of sod-podzolic and gray forest soils, as well as leached chernozem. Experiments to assess water stability were carried out using the method of “blades.” It is based on the dissection of linearly arranged aggregates, which were preliminarily moistened in vacuum to values close to saturation. The energy of hydrophobic bonds depends on the temperature; therefore, the influence of temperature on the value of the determined water stability was studied. Experiments showed that, as the temperature increases, the water stability of aggregates stored in the wet state increases from the moment of selection and decreases as the temperature increases. This indicates the leading role of hydrophobic bonds in the formation of water stability. As for the samples dried to an air-dry state, moistened again, and kept wet for more than two weeks, no temperature dependence of the water stability has been found. Taking into account that the strength of hydrophobic bonds increases with increasing temperature, while that of hydrophilic bonds decreases, the obtained data immutability of water stability can be explained if we assume the joint participation both hydrophobic and hydrophilic bonds in water stability of soil samples that have passed through the stage of drying to an air-dry state. In fact, these results indicate a strong change in the structural organization of soils during drying.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The presence of an agronomically valuable soil structure is an important production feature that gives some advantages: optimal density, which facilitates the germination of seeds and the development of plants from them [1], and favorable water–air and thermal conditions for the development of plants [2, 3]. However, the very presence of the soil structure is determined by its water stability, i.e., the number and strength of intra-aggregate bonds [4], which ensure the existence of soil aggregates.
It has been established [4, 5] that the aggregate composition of soils and the water stability of aggregates are determined by the organic matter in the soil, but there is still no satisfactory hypothesis explaining the mechanisms of this relationship.
There exists the opinion [6] that the water stability of soils is determined by the number (density) of hydrophobic bonds existing between soil particles. According to the mechanism suggested in [7, 8], hydrophilic regions of humic substances (HSs) interact with clay minerals, while hydrophobic regions interact with each other binding soil particles in the aggregate and providing water stability.
There exists another opinion [9, 10] about the mechanism of formation of water-stable aggregates: the content of hydrophilic HS components is increased in the most aggregated soils.
Thus, at the moment there are no common ideas about the types of relationships that form water sta-bility.
The aim of this study is to reveal the nature of the bonds (hydrophilic or hydrophobic) that ensure soil water stability.
The solution of this problem was realized by studying the effect of temperature on the change in water stability and soil aggregates. This method was chosen because of the well-known fact that the strength of hydrophilic bonds decreases with increasing temperature while the strength of hydrophobic bonds increases [6, 11].
We used agro-soddy-podzolic soil (Moscow region), gray forest soil (Tula region), and leached chernozem (Orel region).
Samples of the natural moisture content (0.7–0.8 field moisture capacity) were studied. After sampling in the field, the samples were placed in closed containers. To prevent water loss, the containers were kept in bags, where the humidity was maintained close to 100%. Packages with containers were stored at 25°C. The same samples were also used in this work, dried to an air-dry state, then moistened to 0.7–0.8 field moisture capacity and kept at this humidity for at least two weeks.
In our research we used the “blade” method based on the cutting of aggregates close to water saturation with a blade and determining the limiting stress of their destruction [12]. When preparing samples for research, soils were sifted through sieves, separating a fraction of 4.5–5 mm.
During the measurement, the aggregates were placed in a cassette consisting of three pairs of aluminum angles fixed so that the angle was oriented in the direction of gravity. Cotton wicks were placed at the bottom of the corner.
Fourteen soil aggregates were placed on wicks in aluminum corners so that they touched each other. Air was removed from the aggregates by evacuation for 15 min at a rarefaction of 15 kPa.
After removing the air from the aggregates, the cassette was moved into a desiccator so that the wicks came into contact with water, and the aggregates in vacuum were moistened through the capillaries of wicks to values close to saturation. Due to the unequal wettability of aggregates of different soil types, the time of capillary wetting was selected individually for each of them. For example, for samples of chernozem, the moistening time was 30 min; for gray forest and soddy-podzolic soils, it was 15 min.
After the aggregates were moistened in the vacuum, the cassette was removed from the desiccator and placed in a container with water located on the scales so that the wicks under the aggregates ensured that they were saturated with water, which was achieved at the vacuum stage.
Then, a device was placed on linearly arranged units, which consisted of two parallel blades fixed on a platform, on which a cup with a measuring scale was installed. By adding sand to the cup, the load on the aggregates was increased, which was fixed with the help of weights.
In order to standardize the measured data, the ultimate fracture resistance of the aggregates was calculated. The experimentally determined load in grams was expressed in millinewtons (mN) per unit.
It should be noted that the correlation coefficient between the stability values of water-saturated aggregates obtained using the “blades” method and water stability using the wet screening method is over 85% [12], which makes it possible to use the blade method not only to assess the mechanical strength of aggregates, but also their water stability.
When studying the effect of temperature on the water stability of the soil structure, a cassette with samples (after degassing and capillary wetting of the aggregates) was placed under an infrared lamp for heating or in a refrigerator for cooling. Prevention of drying of the aggregates was achieved by maintaining their capillary contact with water through the wicks. Measurement of water stability was carried out simultaneously with the determination of temperature.
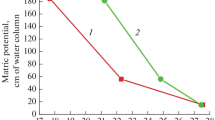
At the first stage of the study, soil samples were studied that were not subjected to drying to an air-dry state. It was found (Figs. 1–3) that the water stability of aggregates of the initial samples of all soil types increased with increasing temperature. When the aggregates cooled to room temperature, their water stability decreased to the initial values. Water stability decreased during the measurement of water stability and soil aggregates at low temperatures. These results confirmed the assumption that hydrophobic bonds underlie the mechanism of water stability in soils not subjected to drying.
At the second stage of this work, the influence of temperature on water stability of the aggregates, which were preliminarily dried to an air-dry state,Footnote 1 was determined. It is seen clearly that this property of all the studied soils did not correspond to the results obtained for the original soils (neither quantitatively nor even qualitatively): water stability remained almost unchanged (Fig. 4).
Taking into account that the strength of hydrophobic bonds increases with increasing temperature, while that of hydrophilic bonds decreases, the measured data on the invariance of water stability can be explained if we assume that hydrophobic and hydrophilic bonds participate jointly in the water stability of soil samples that have passed through the stage of drying to an air-dry state. Actually, these results indicate a strong change in the structural organization of soils during drying and the incorrect use of samples that have passed through the stage of drying to determine the water stability of soils.
When analyzing the literature, we noted above that the water stability of soils, on the one hand, is determined by hydrophobic bonds, and, on the other hand, it has been shown in the literature [13, 14] that the labile (hydrophilic) components of humus play an important role. There is no reason to deny the correctness of the data. Therefore, it can be argued that, in order to increase the water stability, it is necessary to have hydrophobic and hydrophilic compounds of humic substances in the soil, which ensure the presence of both types of bonds in the soil.
This can be explained on the basis of the mechanism of water stability described in [15], which includes the formation of hydrophobic bonds between supramolecular formations of humic substances, that is, fractal clusters (F-clusters) having a dendritic structure. The strength of the bond between two F-clusters is determined by the number of hydrophobic bonds between them: the more bonds, the higher the stability. Strong bonds between F-clusters can arise if they interact with each other not through point hydrophobic contacts on the surface of individual “branches,” but through many hydrophobic contacts that arise when several “branches” are linked. Such interaction is possible only in the presence of hydrophilic regions with ionic atmospheres acting as protectors. They, like similarly charged magnets, repel the “branches” of F-clusters from each other; thereby creating a “corridor” that ensures a deeper interpenetration of these “branches.” As a result, instead of single hydrophobic contacts between individual “branches” of F-clusters, multiple hydrophobic contacts arise between them, which significantly increase the water stability. Otherwise, the interaction between F-clusters occurs through a small number of points on their surface, which cannot ensure high water stability of soils.
CONCLUSIONS
(1) An increase in the water stability of soil aggregates with increasing temperature was found in samples not subjected to drying. This indicates the leading role of hydrophobic bonds, since their energy also increases with increasing temperature.
(2) No effect of temperature on water stability was found on soil samples that passed the stage of drying to an air-dry state. To explain these results, it was suggested that hydrophobic and hydrophilic bonds act oppositely during the drying of soil samples. At the same time, these bonds are equal in total energy; hence, they begin to balance each other and the water stability value remains unchanged.
(3) The results indicate a strong change in the structural organization of soil samples during drying and incorrect determination of the water stability for these samples.
Change history
25 January 2024
An Erratum to this paper has been published: https://doi.org/10.1134/S1028334X2306003X
Notes
According to the requirements of state standard GOST 58595-2019. Soils. Sample selection.
REFERENCES
J. R. Lamichhane, P. Debaeke, C. Steinberg, M. P. You, M. J. Barbetti, and J. N. Aubertot, Plant Soil 432, 1–28 (2018).
C.-A. Houdeshell, R. C. Graham, P. F. Hendrix, and A. C. Peterson, Geoderma 320, 201–208 (2018).
J. Mao, K. G. J. Nierop, S. C. Dekker, L. W. Dekker, and B. Chen, J. Soils Sediments 19, 171–185 (2019).
E. I. Nikolaeva, Candidate’s Dissertation in Biological Science (MSU, Moscow, 2016.).
L. V. Verchot, L. Dutaur, K. D. Shepherd, and A. Albrecht, Geoderma 161 (3–4), 182–193 (2011).
E. S. Vogelmann, J. M. Reichert, J. Prevedello, G. O. Awe, and J. Mataix-Solera, Catena 110, 24–31 (2013).
E. Yu. Milanovsky, Soil Humic Matter as Natural Hydrophobic–Hydrophilic Compounds (GEOS, Moscow, 2009) [in Russian].
E. V. Shein and E. Yu. Milanovskii, Eurasian Soil Sci. 36 (1), 51–59 (2003).
A. A. Shinkarev, L. V. Mel’nikov, and T. E. Zainullin, Eurasian Soil Sci. 32 (3), 317–323 (1999).
A. A. Shinkarev and E. B. Perepelkina, Eurasian Soil Sci. 30 (2), 135–142 (1997).
M. C. Rowley, S. Grand, and E. P. Verrecchia, Biogeochemistry 137 (1–2), 27–49 (2018).
D. A. Ushkova, U. A. Konkina, I. V. Gorepkin, D. I. Potapov, E. V. Shein, and G. N. Fedotov, Eurasian Soil Sci. 56 (2), 177‒184 (2023).
B. M. Kogut, Eurasian Soil Sci. 31 (7), 721‒729 (1998).
B. M. Kogut, Eurasian Soil Sci. 36 (3), 283‒291 (2003).
S. A. Shoba, E. V. Shein, D. A. Ushkova, T. A. Gracheva, O. A. Salimgareeva, and G. N. Fedotov, Dokl. Earth Sci. 507 (2, Suppl.), S340‒S344 (2023).
Funding
This work was carried out within the framework of a State Assignment of Moscow State University, project no. 122011800459-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by E. Morozov
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Fedotov, G.N., Shoba, S.A., Ushkova, D.A. et al. Nature of Bonds in the Formation of Water Stability of Soil Aggregates. Dokl. Earth Sc. 513, 1390–1393 (2023). https://doi.org/10.1134/S1028334X23601967
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1028334X23601967