Abstract
The correlation between the crystal morphological peculiarities of gibbsite and its position in a bauxite-bearing weathering profile is revealed from the example of the Tsentral’noe deposit of the Chadobets Uplift. The free space favorable for the growth of gibbsite increases from bottom to top in various parts of the profile as a result of infiltration metasomatosis and various physicochemical conditions. The higher the position of the rock, the higher its porosity. The size of the gibbsite crystals thus increases, and their morphology becomes more complex upward through the section. The results of thermal analysis showed that the higher the sizes of the gibbsite crystals, the higher the amount of boehmite forms upon its heating. These conclusions will help the technologists to choose the most feasible reworking scheme of bauxites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Aluminum is the main economically important metal in machine and aircraft engineering, the production of drones, construction, and private life. Aluminum is mainly sourced from bauxite ores with major ore-forming gibbsite and boehmite [1]. It is important to identify the mineral composition of bauxites and the morphological features of their Al hydroxides for operation of ore-processing enterprises. Al2O3 production by means of the Bayer process (leaching of bauxite during the interaction with alkali-aluminate solution and further extraction of Al hydroxides from the solution) depends significantly on the ore mineral composition. The easiest reactions occur upon processing of gibbsite bauxite. In this case, the dimensional effect becomes important—the dependence of the properties of a substance on its dispersion. The dispersion and structural peculiarities of gibbsite affect its solubility in the technological cycle. Part of gibbsite transits to the boehmite under increasing temperature [2]. The reworking of boehmite bauxite requires higher temperature and pressure [3].
Many researchers have studied thermal transformations of Al hydroxides upon heating [4–7]. As a result, a number of contradicting conclusions has been made. This is likely because of the different experimental conditions and methods for determination of the heat transformations. The initial stage of thermal decomposition of gibbsite includes the diffusion of protons and the reaction with hydroxyl ions and the formation of water. This process eliminates the linked forces between the layers of the gibbsite structure and causes changes in the chemical composition and density inside the layer [8]. The transformation of gibbsite to boehmite requires hydrothermal conditions in the crystal that maintain the excessive water vapor pressure inside the crystallites. During the initial transformation of gibbsite to boehmite, the crystal surface is characterized by the formation of an impermeable layer. The release of water from the internal part of the crystal stops, which is favorable for the formation of boehmite [9]. This is confirmed by experimental studies [10]. In other models, the transformation of gibbsite to boehmite starts directly inside the crystal and both gibbsite and the envelope around boehmite, which is formed inside, slows down the release of water [11].
A quantitative estimation of the gibbsite content in bauxite ores with an experimental error of ±1% is offered in a series of papers dedicated to the methods of thermal analysis. The bauxites almost always contain Al2O3-free (mostly Fe) minerals. Almost all accessory minerals excluding TiO2 in the form of anatase (or rutile) can be found by the thermal method in bauxites [9, 10].
It should be taken into account upon the quantitative analysis of the gibbsite content in bauxite by thermal analysis that this mineral partly transits to boehmite upon heating. Some researchers consider that gibbsite loses only 2.75 water molecules upon dehydration, whereas the remaining 0.25 molecules are removed upon dehydration of boehmite, which formed upon dehydroxylization of gibbsite [12, 13]. It has also been stated that gibbsite is decomposed to boehmite irrespective of the size distribution of particles [14].
To resolve these contradictions, we studied the mineral composition of bauxites and paragenetic assemblages depending on their position in the weathering profile, in particular, the features of the crystal morphology and sizes of gibbsite crystals using synchronous thermal analysis (STA). The phase composition of bauxite and the crystal morphology were confirmed by X-ray diffractometry (XRD) and scanning electron microscopy (SEM), respectively.
GEOLOGICAL BACKGROUND
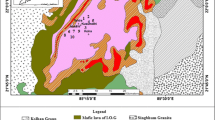
We studied bauxites from different zones of the lateritic profile of the Tsentral’noe deposit on the Chadobets Uplift, which is located in the southwestern part of the ancient Siberian Platform in the Angara–Podkamennaya Tunguska interfluve. The cupola uplift is a brachyanticline structure, which is complicated by two inliers–cores: northern (Terin) and southern (Chuktukon). They are composed of Precambrian rocks with stocks, dikes, and sills of alkaline ultramafic rocks, kimberlite pipes, and carbonatite bodies, which represent the entire Chadobets Complex of alkaline ultramafic rocks. The formation of any weathering crust is always accompanied by partial synchronous denudation and redeposition of weathering products with a consecutive deposition of layers of layered silicates, bauxitic clays, and sedimentary bauxites [15, 16]. Samples were collected and labeled from bottom to top (Fig. 1).
ANALYTICAL METHODS
The samples were crushed to fractions of 0.071 mm in size using an agate mill. The chemical composition of bauxites was analyzed using X-ray fluorescent analysis (XFA) on an Axios Panalytical X-ray fluorescent spectrometer (the Netherlands) equipped with an X-ray tube of 4 kW and a Rh anode. Irrespective of the valent state, the total Fe and S contents are shown as the total Fe2O3 and SO3 content, respectively, which is a peculiarity of the method.
The XRD was conducted on an Ultima-IV diffractometer (Rigaku, Japan) under CuKα1 radiation, a voltage of 40 kV, a beam current of 80 mA, a graphite monochromator, continuous scanning, a scanning rate of 8°/min, a slit of DS = SS = 1°, an ambient temperature of 18°C, and a humidity of 30%.
STA was conducted on a STA 449 F1 Jupiter Netzsch device (Germany) with a record rate of 10°/min in the air atmosphere in crucibles with closed caps up to a temperature of 1050°C. The weight of the sample was ~40 mg. Samples of similar weight were registered in identical conditions.
The electron microscopic studies were carried out on a CamScan 4 SEM (Cambridge, Great Britain) equipped with a LINK-860 energy-dispersive spectrometer (EDS).
RESULTS
X-ray Fluorescent Analysis
According to XFA (Table 1), the SiO2 content of bauxites is 1.03–1.80 wt %. The TiO2, Al2O3, and Fe2O3 contents widely vary: 1.36–7.97, 19.08–48.48, and 17.71–53.78 wt %, respectively. The Na2O and K2O contents are similar (0.01–0.11 wt %), and the P2O5 content is 0.33–0.55 wt %.
X-ray Diffractometry
The analysis showed that the major minerals in all samples include gibbsite, boehmite, hematite, and quartz. The most important and characteristic reflections for these minerals are 4.82 and 4.34 Å for gibbsite, 4.18, 2.69, and 2.45 Å for goethite, 2.69, 1.69, and 2.51 Å for hematite, 3.34, 4.25, and 1.81 Å for quartz, and 3.51, 1.89, and 2.37 Å for anatase.
Synchronous Thermal Analysis
Thermogravimetric (TG), differential TG (DTG), and differential scanning calorimetry (DSC) curves were used to reveal the reactions that occur upon thermal treatment of bauxite samples.
The endothermal effects typical of gibbsite, goethite, and boehmite are registered in DSC curves of all bauxite samples (Figs. 2a–2f). Heating of gibbsite to a temperature of 220‒450°C leads to an endothermal effect on DSC curves related to the release of the main volume of water from gibbsite. At the same time, this leads to the formation of some amount of an intermediate boehmite: γ-Al(OH)3 → AlO(OH) + H2O. Dehydration and decomposition of boehmite occurs at a temperature of 400‒600°C (2AlO(OH) → Al2O3 + H2O), which is reflected as an endothermal effect in this temperature range on the DSC curve. The exothermal effect in the temperature range of 950‒1200°C is related to the phase transition of γ-Al2O3 to α-Al2O3 (corundum).
Dehydration of goethite occurs in the temperature range of 300‒400°C. The mineral loses 10% of hydroxyl groups.
The mineral composition of samples from the bauxite-bearing weathering profile (%) is therefore caused by a correlation between XFA, XRD, and the thermal methods (Table 2). No boehmite was found in primary bauxite samples, which consist of gibbsite, goethite, hematite, quartz, and anatase.
Scanning Electron Microscopy
The size and morphology of gibbsite particles was analyzed using SEM. In total, ten samples collected at the same level were studied. The SEM images are shown in Fig. 3.
Study of the size of gibbsite crystals depending on its position in the bauxite-bearing weathering profile requires reliable determination of the gibbsite content in the rock. The analysis of the mineral composition and the amount of boehmite formed upon heating in samples will be demonstrated with the example of sample 1. Based on XRD, it consists of gibbsite (51 wt %), goethite (41 wt %), and hematite (4 wt %). According to XFA, the sample contains 19.08 wt % Al2O3 and 53.78 wt % Fe2O3. If we take into account that gibbsite contains 65.4 wt % Аl2O3 and 34.6 wt % Н2O, then the calculated gibbsite content in the rock (g) should be
To determine the quantitative gibbsite content in the sample by thermal analysis, it is necessary to take into account the goethite content of the rock, because its dehydration occurs in the same temperature range as gibbsite and their peaks overlap. The endothermal effect on the DSC curve related to dehydroxylization of goethite occurs in the range of 200‒400°С with the maximum at a temperature of 351.3°С. The XFA-based Fe2O3 content is 53.78 wt % including 10% hematite. If we take into account that goethite contains 90 wt % Fe2O3 and 10 wt % Н2O, then theoretically the calculated goethite content in the rock is
This is in agreement with the XRD results. Thus, 4.9 wt % H2O is released during dehydroxylization of goethite.
Dexydroxylization of gibbsite also occurs in the temperature range of 200‒400°С with a maximum at 314.7°С. It is evident from the TG curve that the temperature range of 200‒400°С is favorable for the release of 14.7 wt % H2O, 4.9 wt % of which belongs to goethite; thus, 9.8% H2O is released during dexydroxylization of gibbsite.
Pure gibbsite contains 34.6% hydroxyl groups, which allows the calculation of this mineral on the basis of weight loss upon heating by the TG curves:
This gibbsite content of rock, however, contradicts the XFA results, according to which the gibbsite content should be 29.2%. The difference of 1.4% is explained by the fact that some amount of gibbsite is not dewatered upon heating and is transformed to boehmite (1.4%), dehydroxylization of which occurs in the temperature range of 400‒600°С. It is evident from the TG curve that 0.4% of H2O is released in this range. If we take into account that boehmite contains 85 wt % Аl2O3 and 15 wt % Н2O, then the calculated content of boehmite that forms upon heating is
Similarly using the XRA, XRD, and thermal data, we refined the mineral composition and determined the amounts of boehmite formed upon heating in other samples (Table 3).
DISCUSSION
Thermal transformation of gibbsite can be observed by typical peaks on the DSC curve. It is known that the thermal curve in the temperature range of dehydroxylization of gibbsite has no complications in sedimentary bauxite deposits [2]. The crystal sizes in this deposit group are small. An increased size of gibbsite crystals leads to the formation of an additional peak at a temperature of 260°С on the left shoulder of the endothermal effect on DSC curves. According to experimental data, boehmite forms upon heating at this temperature [18, 19]. Table 3 shows the data after interpretation of the DSC curves, which indicate that the higher the position of the rock, the larger the gibbsite crystals and the higher the percent transformed upon heating (Fig. 1, Table 3). The increasing endothermal peak (Figs. 2a–2f) and related weight loss on the TG curve occur consecutively in the temperature range of the formation of boehmite in the rock upon heating as the size of gibbsite crystal increases. Boehmite remains stable up to a temperature of 400°C. The dexydroxylization of newly formed boehmite occurs in the temperature range of 400‒600°C [4, 8]. The weight losses in this range consecutively increase from the first sample to the last one. The content of the mineral formed upon heating of gibbsite was determined by TG curves and the formula above (Table 3).
SEM data confirm studies of the sizes of gibbsite particles. Gibbsite has an evident crystal structure and the morphology of its crystals depends on the neighboring crystals of other minerals. The size of gibbsite crystals increases upward through the section from 3 to 30 µm; their morphology becomes more complex, and twin crystals are observed.
CONCLUSIONS
The mineral composition of bauxites and the crystal morphological properties of gibbsite from various parts of the bauxite-bearing weathering profile of the Tsentral’noe deposit were identified and studied. The size of the gibbsite crystals increases upward through the profile. The lower part of the section contains small-grained gibbsite, and the gibbsite crystals become larger upward through the section. Our studies show that the larger the size of the gibbsite crystals, the greater the amount of boehmite formed upon heating (i.e., ore processing). According to STA results of samples taken up through the profile, the weight loss related to dexydroxylization of boehmite increases on the TG curves. Taking into account that the primary samples contain no boehmite, the weight loss in the temperature range of 400‒600°C is related to dehydroxylization of boehmite, which formed upon heating of gibbsite. The amount of an intermediate product depends on the crystal sizes of the primary gibbsite. These observations are consistent with SEM study of bauxites.
Zonation of the lateritic weathering crusts is a result of infiltration metasomatosis and various physicochemical conditions in various parts of the profile. The higher the position of the rock, the higher its porosity. The increase in free space promotes an increasing size of the gibbsite crystals.
The STA is one of main methods of study of the mineral composition of bauxites. It is especially useful for determination of the qualitative characteristics related to the sizes of gibbsite crystals. This is an important characteristic of ore, which affects the choice of a rational processing scheme of bauxites. STA application in the study of bauxites supplements the identification of minerals of Al mono- and trihydrates and supports the change in their dimensions during the evolution.
Change history
21 April 2024
An Erratum to this paper has been published: https://doi.org/10.1134/S1028334X23070036
REFERENCES
A. V. Lapin and A. V. Tolstov, Minerageny of Weathering Crusts of Carbonatites (Moscow, 2011) [in Russian].
N. M. Boeva, M. A. Makarova, E. S. Shipilova, A. D. Slukin, F. P. Mel’nikov, O. V. Karimova, and N. S. Bortnikov, Dokl. Earth Sci. 507 (1), 871–881 (2022). https://doi.org/10.1134/S1028334X22600700
S. P. Mehrotra, T. C. Alex, G. Greifzu, and R. Kumar, Trans. Indian Inst. Met. India 69 (1), 51–59 (2015). https://doi.org/10.1007/s12666-015-0633-6
T. Sato, J. Therm. Anal. 32, 61–70 (1987).
Y. Wang, S. Xing, Y. Zhang, Z. Li, Y. Ma, and Z. Zhang, J. Therm. Anal. Calorim. 122, 917–927 (2015). https://doi.org/10.1007/s10973-015-4742-6
M. Laskou, G. Margomenou-Leonidopoulou, and V. Balek, J. Therm. Anal. Calorim. 84, 141–145 (2006). https://doi.org/10.1007/s10973-005-7126-5
Z. D. Zivkovik and D. Blecic, J. Therm. Anal. 33, 413–419 (1988).
R. L. Frost, J. T. Kloprogge, S. C. Russel, and J. L. Szetu, Appl. Spectrosc. 53 (4), 423–434 (1999).
R. Naumann, K. Kohnke, J. Paulik, and F. Paulik, Thermochim. Acta 64 (1–2), 15–26 (1983). https://doi.org/10.1016/0040-6031(83)80124-5
J. Rouquerol, F. Rouquerol, and M. Ganteaume, J. Catal. 36, 99–110 (1975). https://doi.org/10.1016/0021-9517(75)90014-7
C. M. Earnest, Appl. Chem., No. 1, 40–54 (2019). https://doi.org/10.33513/ACBC/1901-06
J. Paulik and F. Paulik, Simultaneous Thermoanalytical Examinations by Means of the Derivatograph (Elsevier Sci. Publ., Amsterdam, 1981).
V. Z. Baranyai, I. Szücs, and Y. F. Kristál, Stud. Univ. Babes-Bolyai, Chem. 60 (2/1), 27–44 (2015).
J. M. R. Mercury, P. Pena, A. H. De Aza, D. Sheptyakov, and X. Turrillas, J. Am. Ceram. Soc. 89 (12), 3728–3733 (2006).
V. I. Mamedov, N. M. Boeva, M. A. Makarova, E. S. Shipilova, and Ph. P. Melnikov, Minerals, No. 12, 389 (2022). https://doi.org/10.3390/min12030389
V. I. Mamedov, M. A. Makarova, N. M. Boeva, D. A. Vnuchkov, and N. S. Bortnikov, Geol. Ore Deposits 63 (6), 599 (2021). https://doi.org/10.1134/S1075701521050044
N. M. Boeva, M. A. Makarova, E. S. Shipilova, A. D. Slukin, S. V. Soboleva, E. A. Zhegallo, L. V. Zaitseva, and N. S. Bortnikov, Dokl. Earth Sci. 504 (2), 353–362 (2022). https://doi.org/10.1134/S1028334X22060046
S. R. Egorova, A. N. Mukhamed’yarova, O. V. Nesterova, Y. Zhang, J. D. Skibina, and A. A. Lamberov, Coatings 8, 1–30 (2018). https://doi.org/10.3390/coatings8010030
M. Authier-Martin, G. Forté, S. Ostap, and J. See, J. Miner., Met. Mater. Soc. 53, 36–40 (2001).
ACKNOWLEDGMENTS
The authors are grateful to A.D. Slukin for samples for study and E.A. Zhegallo for SEM images.
Funding
This work was supported by a state contract of the Institute of Geology of Ore Deposits, Petrography, Mineralogy, and Geochemistry, Russian Academy of Sciences, project no. 121041500220-0. The analytical studies were conducted in the Analitika Center for Collective Use, Institute of Geology of Ore Deposits, Petrography, Mineralogy, and Geochemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by I. Melekestseva
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boeva, N.M., Bortnikov, N.S. The Dimensional Effect and Crystal Morphological Peculiarities of Gibbsite in a Bauxite-Bearing Weathering Crust. Dokl. Earth Sc. 510, 269–275 (2023). https://doi.org/10.1134/S1028334X23600196
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1028334X23600196