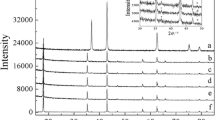
Abstract
Many lithium intercalation compounds, which have successful applications as lithium-ion battery electrode materials, are used not in individual state, but as a part of specially organized composites containing also auxiliary components distributed over the surface of intercalation-material particles, as well as in the interparticle space. The applied modifier-substances affect such characteristics of intercalation materials as capacity, its reversibility, and persistence during long-term cycling in the charge–discharge mode, as well as with varying the electrode current and potential ranges. In this work, the behavior of a modifying agent, belonging to the class of compounds known in the literature as MAX phases, is studied in detail in the composition of an electroactive composite. The MAX-phase agents have general formula Mn + 1AXn, where M is the transition metal, A is the element of the Periodic Table III–VI groups, and X is C or N. The temperature required for the Ti3SiC2 compound synthesis is close to 1500°C. We succeeded in reducing the temperature by means of preliminary mechanochemical treatment of the reagents’ mixture. The action mechanism of the Ti3SiC2-modifier is considered in comparison with similar models proposed in the literature. Comparison of the characteristics of composite materials with different Ti3SiC2-content and different types of modified intercalation compounds (substrates) showed a positive effect of the modifier both on the kinetics of electrode processes and the rate of degradation of the materials’ capacitive characteristics.









Similar content being viewed by others
REFERENCES
Conway, B.E., Transition from “Supercapacitor” to “Battery” Behavior in Electrochemical Energy Storage, J. Electrochem. Soc., 1991, vol. 138, p. 1539.
Eftekhari, A., Low voltage anode materials for lithium-ion batteries, Energy Storage Mater., 2017, vol. 7, p. 157.
Simon, P., Gogotsi, Y., and Dunn, B., Where Do Batteries End and Supercapacitors Begin? Science, 2014, vol. 343, p. 1210.
Xu, J., Zhao, M.-Q., Wang, Y., Yao, W., Chen, C., Anasori, B., Sarycheva, A., Ren, C.E., Mathis, T., Gomes, L., Zhenghua, L., and Gogotsi, Y., Demonstration of Li-Ion Capacity of MAX Phases, ACS Energy Lett., 2016, vol. 1, p. 1094.
Huang, H., Yin, S.-C., Kerr, T., Taylor, N., and Nazar, L.F., Nanostructured Composites: A High Capacity, Fast Rate Li3V2(PO4)3/Carbon Cathode for Rechargeable Lithium Batteries, Adv. Mater., 2002, vol. 14, p. 1525.
Gaubicher, J., Wurm, C., Goward, G., Masquelier, C., and Nazar, L., Rhombohedral Form of Li3V2(PO4)3 as a Cathode in Li-Ion Batteries, Chem. Mater., 2000, vol. 12, p. 3240.
Ivanishchev, A.V., Churikov, A.V., and Ushakov, A.V., Lithium transport processes in electrodes on the basis of Li3V2(PO4)3 by constant current chronopotentiometry, cyclic voltammetry and pulse chronoamperometry, Electrochim. Acta, 2014, vol. 122, p. 187.
Ivanishchev, A.V., Churikov, A.V., Ivanishcheva, I.A., and Ushakov, A.V., Lithium diffusion in Li3V2(PO4)3-based electrodes: a joint analysis of electrochemical impedance, cyclic voltammetry, pulse chronoamperometry, and chronopotentiometry data, Ionics, 2016, vol. 22, p. 483.
Ivanishchev, A.V., Ushakov, A.V., Ivanishcheva, I.A., Churikov, A.V., Mironov, A.V., Fedotov, S.S., Khasanova, N.R., and Antipov, E.V., Structural and electrochemical study of fast Li diffusion in Li3V2(PO4)3-based electrode material, Electrochim. Acta, 2017, vol. 230, p. 479.
Babbar, P., Ivanishchev, A., Churikov, A., and Dixit, A., Electrochemical behavior of carbonic precursor with Na3V2(PO4)3 nanostructured material in hybrid battery system, Ionics, 2017, vol. 23, p. 3067.
Ushakov, A.V., Makhov, S.V., Gridina, N.A., Ivanishchev, A.V., and Gamayunova, I.M., Rechargeable lithium-ion system based on lithium–vanadium(III) phosphate and lithium titanate and the peculiarity of it functioning, Monatsh. Chem., 2019, vol. 150, p. 499.
Sun, C., Rajasekhara, S., Dong, Y., and Goodenough, J.B., Hydrothermal Synthesis and Electrochemical Properties of Li3V2(PO4)3/C-Based Composites for Lithium-Ion Batteries, ACS Appl. Mater. Inter., 2011, vol. 3, p. 3772.
Chen, Y., Zhao, Y., An, X., Liu, J., Dong, Y., and Chen, L., Preparation and electrochemical performance studies on Cr-doped Li3V2(PO4)3 as cathode materials for lithium-ion batteries, Electrochim. Acta, 2009, vol. 54, p. 5844.
Yao, J., Wei, S., Zhang, P., Shen, C., Aguey-Zinsou, K.-F., and Wang, L., Synthesis and properties of Li3V2 – xCex(PO4)3/C cathode materials for Li-ion batteries, J. Alloy. Compd., 2012, vol. 532, p. 49.
Yuan, W., Yan, J., Tang, Z., Sha, O., Wang, J., Mao, W., and Ma, L., Mo-doped Li3V2(PO4)3/C cathode material with high rate capability and long term cyclic stability, Electrochim. Acta, 2012, vol. 72, p. 138.
Bini, M., Ferrari, S., Capsoni, D., and Massarotti, V., Mn influence on the electrochemical behaviour of Li3V2(PO4)3 cathode material, Electrochim. Acta, 2011, vol. 56, p. 2648.
Ren, M.M., Zhou, Z., Gao, X.P., Peng, W.X., and Wei, J.P., Core-Shell Li3V2(PO4)3@C Composites as Cathode Materials for Lithium-Ion Batteries, J. Phys. Chem. C, 2008, vol. 112, p. 5689.
Pan, A., Liu, J., Zhang, J.-G., Xu, W., Cao, G., Nie, Z., Arey, B.W., and Liang, S., Nano-structured Li3V2(PO4)3/carbon composite for high-rate lithium-ion batteries, Electrochem. Commun., 2010, vol. 12, p. 1674.
Teng, F., Hu, Z.-H., Ma, X.-H., Zhang, L.-C., Ding, C.-X., Yu, Y., and Chen, C.-H., Hydrothermal synthesis of plate-like carbon-coated Li3V2(PO4)3 and its low temperature performance for high power lithium ion batteries, Electrochim. Acta, 2013, vol. 91, p. 43.
Eftekhari, A., LiFePO4/C nanocomposites for lithium-ion batteries, J. Power Sources, 2017, vol. 343, p. 395.
Konarova, M. and Taniguchi, I., Synthesis of carbon-coated LiFePO4 nanoparticles with high rate performance in lithium secondary batteries, J. Power Sources, 2010, vol. 195, p. 3661.
Chong, J., Xun, S., Song, X., Ridgway, P., Liu, G., and Battaglia, V.S., Towards the understanding of coatings on rate performance of LiFePO4, J. Power Sources, 2012, vol. 200, p. 67.
Kam, K.C., Gustafsson, T., and Thomas, J.O., Synthesis and electrochemical properties of nanostructured Li2FeSiO4/C cathode material for Li-ion batteries, Solid State Ionics, 2011, vol. 192, p. 356.
Fujita, Y., Iwase, H., Shida, K., Liao, J., Fukui, T., and Matsuda, M., Synthesis of high-performance Li2FeSiO4/C composite powder by spray-freezing/freeze-drying a solution with two carbon sources, J. Power Sources, 2017, vol. 361, p. 115.
Jeitschko, W. and Nowotny, H., Die Kristallstruktur von Ti3SiC2—ein neuer Komplexcarbid-Typ, Monatsh. Chem., 1967, vol. 98, p. 329.
Barsoum, M.W. and El-Raghy, T., Synthesis and Characterization of a Remarkable Ceramic: Ti3SiC2, J. Am. Ceram. Soc., 1996, vol. 79, p. 1953.
Barsoum, M.W. and El-Raghy, T., The MAX Phases: Unique New Carbide and Nitride Materials: Ternary ceramics turn out to be surprisingly soft and machinable, yet also heat-tolerant, strong and lightweight, Am. Sci., 2001, vol. 89, p. 334.
Högberg, H., Hultman, L., Emmerlich, J., Joelsson, T., Eklund, P., Molina-Aldareguia, J.M., Palmquist, J.-P., Wilhelmsson, O., and Jansson, U., Growth and characterization of MAX-phase thin films, Surf. Coat. Tech., 2005, vol. 193, p. 6.
Sun, Z.M., Progress in research and development on MAX phases: a family of layered ternary compounds, Int. Mater. Rev., 2011, vol. 56, p. 143.
An, J., Liu, C., Guo, R., Li, Y., and Xu, W., Ti3SiC2 Modified LiFePO4/C Cathode Materials with Improved Electrochemical Performance, J. Electrochem. Soc., 2012, vol. 159, p. A2038.
Pampuch, R., Lis, J., Stobierski, L., and Tymkiewicz, M., Solid combustion synthesis of Ti3SiC2, J. Eur. Ceram. Soc., 1989, vol. 5, p. 283.
Cai, G., Guo, R., Liu, L., Yang, Y., Zhang, C., Wu, C., Guo, W., and Jiang, H., Enhanced low temperature electrochemical performances of LiFePO4/C by surface modification with Ti3SiC2, J. Power Sources, 2015, vol. 288, p. 136.
Wu, C., Guo, R., Cai, G., Zhang, C., Yang, Y., Guo, W., Liu, Z., Wan, Y., and Jiang, H., Ti3SiC2 modified Li3V2(PO4)3/C cathode materials with simultaneous improvement of electronic and ionic conductivities for lithium ion batteries, J. Power Sources, 2016, vol. 306, p. 779.
Sun, D., Wu, C., Guo, R., Liu, Z., Xie, D., Zheng, M., Wang, B., Peng, J., and Jiang, H., Enhanced low temperature electrochemical properties of Li3V2(PO4)3/C modified by a mixed conductive network of Ti3SiC2 and C, Ceram. Int., 2017, vol. 43, p. 2791.
Medvedeva, N.I., Enyashin, A.N., and Ivanovskii, A.L., Modeling of the electronic structure, chemical bonding, and properties of ternary silicon carbide Ti3SiC2, J. Struct. Chem., 2011, vol. 52, p. 785.
Xu, Y.-G., Ou, X.-D., and Rong, X.-M., Vacancy trapping behaviors of hydrogen in Ti3SiC2: A first-principles study, Mater. Lett., 2014, vol. 116, p. 322.
Eklund, P., Beckers, M., Jansson, U., Högberg, H., and Hultman, L., The Mn+1AXn phases: Materials science and thin-film processing, Thin Solid Films, 2010, vol. 518, p. 1851.
Kero, I., Tegman, R., and Antti, M.-L., Effect of the amounts of silicon on the in situ synthesis of Ti3SiC2 based composites made from TiC/Si powder mixtures, Ceram. Int., 2010, vol. 36, p. 375.
Grigoryan, A.E., Rogachev, A.S., Sychev, A.E., and Levashov, E.A., SHS and formation of structure in composite materials in three-component Ti–Si–C, Ti‒Si–N, and Ti–B–N systems, Refract. Ind. Ceram., 1999, vol. 40, p. 484.
Goto, T. and Hirai, T., Chemically vapor deposited Ti3SiC2, Mater. Res. Bull., 1987, vol. 22, p. 1195.
Zhimei, S., Yi, Z., and Yanchun, Z., Synthesis of Ti3SiC2 powders by a solid-liquid reaction process, Scripta Mater., 1999, vol. 41, p. 61.
Sun, Z. and Zhou, Y., Fluctuation synthesis and characterization of Ti3SiC2 powders, Mater. Res. Innov., 1999, vol. 2, p. 227.
Arunajatesan, S. and Carim, A.H., Synthesis of Titanium Silicon Carbide, J. Am. Ceram. Soc., 1995, vol. 78, p. 667.
Kellerman, D.G., Gorshkov, V.S., Blinovskov, Ya.N., Grigorov, I.G., Perelyaev, V.A., and Shveikin, V.A., Synthesis and properties of the ternary phase Ti3SiC2, Inorg. Mater., 1997, vol. 33, p. 271.
Yang, S., Sun, Z.M., and Hashimoto, H., Reaction in Ti3SiC2 powder synthesis from a Ti–Si–TiC powder mixture, J. Alloy. Compd., 2004, vol. 368, p. 312.
Goldin, B.A., Istomin, P.V., and Ryabkov, Yu.I., Reduction solid-state synthesis of titanium silicide carbide, Ti3SiC2, Inorg. Mater., 1997, vol. 33, p. 577.
Istomin, P.V., Nadutkin, A.V., Ryabkov, Yu.I., and Goldin, B.A., Preparation of Ti3SiC2, Inorg. Mater., 2006, vol. 42, p. 250.
Zou, Y., Sun, Z.M., Tada, S., and Hashimoto, H., Effect of Al addition on low-temperature synthesis of Ti3SiC2 powder, J. Alloy. Compd., 2008, vol. 461, p. 579.
Liang, B., Han, X., Zou, Q., Zhao, Y., and Wang, M., TiC/Ti3SiC2 composite prepared by mechanical alloying, Int. J. Refract. Met. H., 2009, vol. 27, p. 664.
Cui, Y.R., Xu, Y.H., Xu, S.C., Li, X.M., and Yang, J., Synthesis of High Purity Ti3SiC2 Powder by Vacuum Sintering, Mater. Sci. Forum., 2009, vols. 620–622, p. 331.
Avvakumov, E.G., Mechanical Activation Methods of Chemical Processes (in Russian), Novosibirsk: Nauka, 1989.
Chen, Y., Zhang, D., Bian, X., Bie, X., Wang, C., Du, F., Jang, M., Chen, G., and Wei, Y., Characterizations of the electrode/electrolyte interfacial properties of carbon coated Li3V2(PO4)3 cathode material in LiPF6 based electrolyte, Electrochim. Acta, 2012, vol. 79, p. 95.
Zhang, S., Wu, Q., Deng, C., Liu, F.L., Zhang, M., Meng, F.L., and Gao, H., Synthesis and characterization of Ti–Mn and Ti–Fe codoped Li3V2(PO4)3 as cathode material for lithium ion batteries, J. Power Sources, 2012, vol. 218, p. 56.
Yin, S.-C., Grondey, H., Strobel, P., Anne, M., and Nazar, L.F., Electrochemical Property: Structure Relationships in Monoclinic Li3 – yV2(PO4)3, J. Am. Chem. Soc., 2003, vol. 125, p. 10402.
Zhang, L.-L., Liang, G., Peng, G., Zou, F., Huang, Y.-H., Croft, M.C., and Ignatov, A., Significantly Improved Electrochemical Performance in Li3V2(PO4)3/C Promoted by SiO2 Coating for Lithium-Ion Batteries, J. Phys. Chem. C, 2012, vol. 116, p. 12401.
Markevich, E., Sharabi, R., Gottlieb, H., Borgel, V., Fridman, K., Salitra, G., Aurbach, D., Semrau, G., Schmidt, M.A., Schall, N., and Bruenig, C., Reasons for capacity fading of LiCoPO4 cathodes in LiPF6 containing electrolyte solutions, Electrochem. Commun., 2012, vol. 15, p. 22.
Markovsky, B., Rodkin, A., Cohen, Y.S., Palchik, O., Levi, E., Aurbach, D., Kim, H.-J., and Schmidt, M., The study of capacity fading processes of Li-ion batteries: major factors that play a role, J. Power Sources, 2003, vols. 119–121, p. 504.
Ahrens, L.H., The use of ionization potentials Part 1. Ionic radii of the elements, Geochim. Cosmochim. Ac., 1952, vol. 2, p. 155.
Svitan’ko, A., Scopets, V., Novikova, S., and Yaroslavtsev, A., The effect of composite formation with oxides on the ion conductivity of NASICON-Type LiTi2(PO4)3 and olivine-type LiFePO4, Solid State Ionics, 2015, vol. 271, p. 42.
Iltchev, N., Chen, Y., Okada, S., and Yamaki, J.-I., LiFePO4 storage at room and elevated temperatures, J. Power Sources, 2003, vols. 119–121, p. 749.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 20-03-00381).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
Based on the materials of the report at the 15th International Meeting “Fundamental Problems of Solid State Ionics”, Chernogolovka, 30.11.–07.12.2020.
Rights and permissions
About this article
Cite this article
Ivanishchev, A.V., Ivanishcheva, I.A., Nam, SC. et al. Electroactive Composites Based on Lithium Intercalation Compounds and Highly Conductive Materials: Methods of Synthesis and Electrochemical Characteristics. Russ J Electrochem 57, 706–720 (2021). https://doi.org/10.1134/S1023193521070053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193521070053