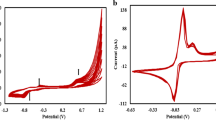
Abstract
Electropolymerization of pyrrole using an anionic sulfosalicylic acid ligand on the surface of electrode followed by overoxidation of membrane is performed to design a new modified electrode (ME) to measure specifically Cu2+ via complexation and intrusion of copper ion into the polymeric membrane. Copper binding property of ligand, overoxidation, and applied potential and polymeric structure of the modified electrodes are factors that encourage binding performance of Cu2+ into polymeric membrane of the electrode and lowering the detection limit. Presence of Cu2+ into the membrane was approved by Energy Dispersive Analysis X-Ray. Preconcentration of the analyte into modified electrode followed by differential pulse anodic stripping voltammetry is used to detect and measure Cu2+ quantitatively. Potentiometric measurements were conducted to test the ability of electrode to act as an ion selective for the direct measurement of Cu2+. Two interfering elements were Ag and Hg, but other elements such as Ni2+, Al3+, Zn2+, Ba2+, Mn2+, Co2+, Cd2+, and Pb2+even with 100 times more concentrated than analyte did not interfere. The electrode showed stable and reproducible surface with RSD less than 0.9%, range of linearity was from 1.0 × 10–8 to 1.0 × 10–3 M with a detection limit of 1.5 × 10–9 M. In the case of potentiometric measurements, RSD = 0.4%, linearity from 1.0 × 10–8 to 1.0 × 10–3 M, detection limit about 4.0 × 10–9 M and response time was 8 to 40 s.












Similar content being viewed by others
REFERENCES
Flato, J.B., Renaissance in polarographic and voltammetric analysis, Anal. Chem., 1972, vol. 44, p. 75A.
Abdollahi, S., Preconcentration and determination of Pb2+ at an AlPO4 containing carbon paste electrode, Anal. Chim. Acta, 1995, vol. 304, p. 381.
Baldwin, R.P. and Thomsen, K.N., Chemically modified electrodes in liquid chromatography detection: a review, Talanta, 1991, vol. 38, p. 1.
Prabhu, S.Y., Baldwin, R.P., and Kryger, L., Preconcentration and determination of lead(II) at crown ether and cryptand containing chemically modified electrodes, Electroanalysis, 1989, vol. 1, p. 13.
Agraz, R., Sevilla, M.T., and Hernandez, L., Chemically modified electrode for the simultaneous determination of trace metals and speciation analysis, Anal. Chim Acta, 1993, vol. 273, p. 205.
Satyanarayana, M., Goud, K.Y., and Gobi, K.V., Conducting polymer-layered carbon nanotube as sensor interface for electrochemical detection of dacarbazine in-vitro, Electrocatalysis, 2017, vol. 8, p. 214.
Asghari, E. and Malekian, S., Improvement of the electrocatalytic performance of platinum-free hierarchical Cu/polypyrrole/NiO anode for methanol oxidation via changing the morphology of polypyrrole sublayer by selfassembled pyrrole monomers and overoxidation, Synth. Met., 2017, vol. 229, p. 57.
Otero, T.F. and Beaumont, S., Chemical sensors from the cooperative actuation of multistep electrochemical molecular machines of polypyrrole: voltammetric study, Sens. Actuators, B, 2017, vol. 253, p. 958.
Price, J.F. and Baldwin, R.P., Preconcentration and determination of ferrocene carboxaldehyde at a chemically modified platinum electrode, Anal. Chem., 1980, vol. 52, p. 1940.
Cheek, G.T. and Nelson, R.F., Applications of chemically modified electrodes to analysis of metal ions, Anal. Lett., 1978, vol. 11, p. 393.
Situ, Bo., Zhao, J., and Lv, W., Naked-eye detection of copper(II) ions by a “clickable” fluorescent sensor, Sens. Actuators, B, 2017, vol. 240, p. 560.
Zhou, X., Li, G., and Yang, P., A switching sensor of C–H bond breakage/formation regulated by mediating copper(II)’s complexation, Sens. Actuators, B, 2017, vol. 242, p. 56.
Farajollahi, M., Woehling, V., and Plesse, C., Self-contained tubular bending actuator driven by conducting polymers, Sens. Actuators, A, 2016, vol. 249, p. 45.
Li, P., Gao, Z., and Xu, Y., Determination of trace amounts of silver with a chemically modified carbon paste electrode, Anal. Chim. Acta, 1990, vol. 229, p. 213.
Yan, R., Qiu, S., and Tong, L., Review of progresses on clinical applications of ion selective electrodes for electrolytic ion tests: from conventional ISEs to graphene-based ISEs, Chem. Spec. Bioavailab., 2016, vol. 28, p. 72.
Salles, M.O., Araujo, W.R., and Paixão, T.R.L.C., Development of a molecularly imprinted modified electrode to evaluate phenacetin based on the preconcentration of acetaminophe, J. Braz. Chem. Soc., 2016, vol. 27, p. 54.
Rahman, S.F., Min, K., and Park, S.H., Selective determination of dopamine with an amperometric biosensor using electrochemically pretreated and activated carbon/tyrosinase/Nafion-modified glassy carbon electrode, Biotechnol. Bioproc. E, 2016, vol. 21, p. 627.
Attia, A.K., Frag, E.Y.Z., and Ahmed, H.E., Validated electroanalytical determination of flavoxate hydrochloride and tolterodine tartrate drugs in bulk, dosage forms and urine using modified carbon paste electrodes, Arab. J. Chem., 2016, vol. 11, p. 483.
Borazjani, M., Mehdinia, A., and Ziaei, E., Enantioselective electrochemical sensor for R-mandelic acid based on a glass carbon electrode modified with multi-layers of biotin-loaded overoxidized polypyrrole and nanosheets of reduced grapheme oxide, Microchim. Acta, 2016, vol. 184, p. 611.
Alizadeh, N., Kamalabadi, M., and Mohammadi, A., Determination of histamine and tyramine in canned fish samples by headspace solid-phase microextraction based on a nanostructured polypyrrole fiber followed by ion mobility spectrometry, Food Anal. Method, 2017, vol. 10, p. 3001.
Zhao, Y., Lv, Z., and Wang, Y., Combination of Fe–Mn based Li-rich cathode materials and conductingpolymer polypyrrole nanowires with high rate capability, Ionics, 2017, vol. 24, p. 51.
Genova, F.K.M., Premalatha, M., and Selvasekarapandian, S., Lithium ionconducting polymer electrolytes based on PVA–PAN doped with lithium triflate, Ionics, 2017, vol. 23, p. 2727.
Oyama, N. and Anson, F.C., Polymeric ligands as anchoring groups for the attachment of metal complexes to graphite electrode surfaces, J. Am. Chem. Soc., 1979, vol. 101, p. 3450.
Oyama, N. and Anson, F.C., Electrostatic binding of metal complexes to electrode surfaces coated with highly charged polymeric films, J. Electrochem. Soc., 1980, vol. 127, p. 247.
Prabhu, S.Y. and Baldwin, R.P., Chemical preconcentration and determination of copper at a chemically modified carbon-paste electrode containing 2,9-dimethyl-1,10-phenanthroline, Anal. Chem., 1987, vol. 59, p. 1074.
Zanganeh, A.R. and Amini, M.K., Polypyrrole-modified electrodes with induced recognition sites for potentiometric and voltammetric detection of copper(II) ion, Sens. Actuators, B, 2008, vol. 135, p. 358.
Beck, F., Braun, P., and Oberst, M., Organic electrochemistry in the solid state-overoxidation of polypyrrole, Bunsen-Ges. Phys.Chem., Ber., 1987, vol. 91, p. 967.
Ge, H.L., Qi, G.J., and Kang, E.T., Study of overoxidized polypyrrole using X-ray photoelectron spectroscopy, Polymer, 1994, vol. 35, p. 504.
Ersoz, A., Gavalas, V.G., and Bachas, L.G., Potentiometric behavior of electrodes based on overoxidized polypyrrole film, Anal. Bioanal. Chem., 2002, vol. 372, p. 786.
Bobacka, J., Ivaska, A., Lewenstam, A., Potentiometric ion sensors, Chem. Rev., 2008, vol. 108, p. 329.
Liu, A.S. and Oliveira, M.A.S., Electrodeposition of polypyrrole films on aluminum from tartrate aqueous solution, J. Braz. Chem. Soc., 2007, vol. 18, p. 143.
Prissanaroon-Ouajai, W., Pigram, P.J., and Jones, R., A sensitive and highly stable polypyrrole-based pH sensor with hydroquinone monosulfonate and oxalate co-doping, Sens. Actuators, B, 2009, vol. 138, p. 504.
Tietje-Girault, J., de León, C.P., and Walsh, F.C., Electrochemically deposited polypyrrole films and their characterization, Surf. Coat. Technol., 2007, vol. 201, p. 6025.
Otero, T.F. and Delarreta, E., Electrochemical control of morphology adherence, appearance and growth of pplprrole films, Synth. Met., 1988, vol. 26, p. 79.
Randjelović, P., Veljković, S., and Stojiljković, N., The beneficial biological properties of salicylic acid, Acta Fac. Med. Nais., 2015, vol. 32, p. 259.
Madan, R.K. and Levitt, J., A review of toxicity from topical salicylic acid preparations, J. Am. Acad. Dermatol., 2014, vol. 70, p. 788.
Praveen, K., Madhavi, D.S.S., and Anil Kumar, K., Coordination chemistry of salicylic acid, Int. J. Eng. Sci. Inv., 2016, vol. 05, p. 08.
Yin, M.C., Ai, C.C., and Yuan, L.J., Synthesis, structure and luminescent property of a binuclear terbium complex [Tb2(Hsal)8(H2O)2][(Hphen)2] · 2H2O, J. Mol. Struct., 2004, vol. 691, p. 33.
Chen, H.J., Lin, Z.Y., and Li, M.Y., A new, efficient, and inexpensive copper(II)/salicylic acid complex catalyzed Sonogashira-type cross-coupling of haloarenes and iodoheteroarenes with terminal alkynes, Tetrahedron, 2010, vol. 66, p. 7755.
Wenb, J., Zhoua, L., and Jinb, L., Overoxidized polypyrrole/multi-walled carbon nanotubes composite modified electrode for in vivo liquid chromatography-electrochemical detection of dopamine, J. Chrom. B, 2009, vol. 877, p. 1793.
Li, Y., Wang, P., and Wang, L., Overoxidized polypyrrole film directed single-walled carbon nanotubes immobilization on glassy carbon electrode and its sensing applications, Biosens. Bioelectron., 2007, vol. 22, p. 3120.
Khalkhali, R., Price, W.E., and Wallace, G.G., Quartz crystal microbalance studies of the effect of solution temperature on the ion-exchange properties of polypyrrole conducting electroactive polymers, React. Funct. Polym., 2003, vol. 56, p. 141.
Brânzoi, V., Brânzoi, F., and Pilan, L., Electrochemical characterization of polypyrrole modified electrodes doped with large organic anions, Rev. Roum. Chim., 2007, vol. 52, p. 169.
ACKNOWLEDGMENTS
We sincerely thank Dr. Zanganeh and Dr. Faghihian for his precious guidance through all the research processes.
Funding
We would like to acknowledge the Islamic Azad University of Shahreza for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bagheri, A., Hassani Marand, M. Voltammetric and Potentiometric Determination of Cu2+ Using an Overoxidized Polypyrrole Based Electrochemical Sensor. Russ J Electrochem 56, 453–461 (2020). https://doi.org/10.1134/S1023193520060026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193520060026