Abstract
The authors describe an electrochemical method for quantitation of R-mandelic acid (R-MA). A glassy carbon electrode (GCE) was modified by electrochemical deposition of several layers of D-(+)-biotin-loaded overoxidized polypyrrole (OPPy-biot) on nanosheets of reduced graphene oxide. The deposited film was characterized by scanning electron microscopy, differential pulse voltammetry, cyclic voltammetry and electrochemical impedance spectroscopy. The modified GCE is shown to enable stereoselective recognition of R-MA even in the presence of high concentrations of S-mandelic acid (S-MA), best at a voltage of around 1.5 V (vs. Ag/AgCl). The analytical performance of the electrode was studied by differential pulse voltammetry which revealed a linear range that extends over the 5 to 80 mM R-MA concentration range, a 1.5 mM detection limit, and an enanotiospecific characteristic toward R-MA. Under optimum conditions, the method displays high reproducibility and stability. It was applied to the determination of R-MA in a synthetic mixture and no interference was observed by S-MA.

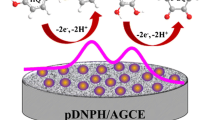
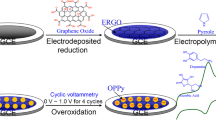
Multi-layered of D-(+)-biotin-loaded overoxidized polypyrrole film on the nanosheets of reduced graphene oxide-modified glassy carbon electrode was fabricated by electrochemical deposition, which are shown to enable enantioselective determination of R-mandelic acid in the presence of high concentration of S-mandelic acid.







Similar content being viewed by others
References
Xu M-H, Lin J, Hu Q-S, Pu L (2002) Fluorescent sensors for the enantioselective recognition of mandelic acid: signal amplification by dendritic branching. J Am Chem Soc 124(47):14239–14246
Bonrath W, Karge R, Netscher T, Roessler F, Spindler F (2009) Biotin–the chiral challenge. CHIMIA International Journal for Chemistry 63(5):265–269
Bhuniya S, Park SM, Kim BH (2005) Biotin-amino acid conjugates: an approach toward self-assembled hydrogelation. Org Lett 7(9):1741–1744
Tripathi A, Kumar A, Pandey PS (2012) Visual chiral recognition of mandelic acid and α-amino acid derivatives by enantioselective gel formation and precipitation. Tetrahedron Lett 53(43):5745–5748
Guo H-S, Kim J-M, Chang S-M, Kim W-S (2009) Chiral recognition of mandelic acid on quartz crystal microbalance by vapor diffused molecular assembly method. J Nanosci Nanotechnol 9(5):2937–2943
Kim BH, Bhuniya S, Park SM (2005) Biotin-amino acid conjugate useful as a Hydrogelator and hydrogel prepared therefrom. Google Patents,
Tu F-Y, Yu L-Y, Yu J-G, Chen X-Q, Fu Q, Jiao F-P, Peng Z-G, Zhang T (2013) Graphene as tunable stationary phase additive for enantioseparation. Nano 8(06):1350069
Zor E, Saf AO, Bingol H, Ersoz M (2014) Voltammetric discrimination of mandelic acid enantiomers. Anal Biochem 449:83–89
Fu Y, Chen Q, Zhou J, Han Q, Wang Y (2012) Enantioselective recognition of mandelic acid based on γ-globulin modified glassy carbon electrode. Anal Biochem 421(1):103–107
Zhang Q, Wang Y, Han Q, Guo L, Huang Y, Fu Y (2013) Enantioselective recognition of Mandelic acid based on hemoglobin and multiwall carbon nanotubes modified electrode. J Electrochem Soc 160(11):B213–B217
Fu Y, Wang L, Chen Q, Zhou J (2011) Enantioselective recognition of chiral mandelic acid in the presence of Zn (II) ions by l-cysteine-modified electrode. Sensors Actuators B Chem 155(1):140–144
Guo H-S, Kim J-M, Chang S-M, Kim W-S (2009) Chiral recognition of mandelic acid by L-phenylalanine-modified sensor using quartz crystal microbalance. Biosens Bioelectron 24(9):2931–2934
Gerard M, Chaubey A, Malhotra B (2002) Application of conducting polymers to biosensors. Biosens Bioelectron 17(5):345–359
Yu H, Jian X, Jin J, Zheng X-c, R-t L, G-c Q (2015) Nonenzymatic sensing of glucose using a carbon ceramic electrode modified with a composite film made from copper oxide, overoxidized polypyrrole and multi-walled carbon nanotubes. Microchim Acta 182(1–2):157–165
Yang J, Cho M, Pang C, Lee Y (2015) Highly sensitive non-enzymatic glucose sensor based on over-oxidized polypyrrole nanowires modified with Ni (OH) 2 nanoflakes. Sensors Actuators B Chem 211:93–101
Carelli D, Centonze D, De Giglio A, Quinto M, Zambonin PG (2006) An interference-free first generation alcohol biosensor based on a gold electrode modified by an overoxidised non-conducting polypyrrole film. Anal Chim Acta 565(1):27–35
Majidi MR, Jouyban A, Asadpour-Zeynali K (2007) Electrocatalytic oxidation of hydrazine at overoxidized polypyrrole film modified glassy carbon electrode. Electrochim Acta 52(21):6248–6253
Majidi MR, Jouyban A, Asadpour-Zeynali K (2005) Genetic algorithm based potential selection in simultaneous voltammetric determination of isoniazid and hydrazine by using partial least squares (PLS) and artificial neural networks (ANNs). Electroanalysis 17(10):915–918
Lin M (2015) A dopamine electrochemical sensor based on gold nanoparticles/over-oxidized polypyrrole nanotube composite arrays. RSC Adv 5(13):9848–9851
Sasso L, Heiskanen A, Diazzi F, Dimaki M, Castillo-León J, Vergani M, Landini E, Raiteri R, Ferrari G, Carminati M (2013) Doped overoxidized polypyrrole microelectrodes as sensors for the detection of dopamine released from cell populations. Analyst 138(13):3651–3659
Pihel K, Walker QD, Wightman RM (1996) Overoxidized polypyrrole-coated carbon fiber microelectrodes for dopamine measurements with fast-scan cyclic voltammetry. Anal Chem 68(13):2084–2089
Li J, Lin X (2007) Simultaneous determination of dopamine and serotonin on gold nanocluster/overoxidized-polypyrrole composite modified glassy carbon electrode. Sensors Actuators B Chem 124(2):486–493
Jiang X, Lin X (2005) Overoxidized polypyrrole film directed DNA immobilization for construction of electrochemical micro-biosensors and simultaneous determination of serotonin and dopamine. Anal Chim Acta 537(1):145–151
Tsai T-C, Han H-Z, Cheng C-C, Chen L-C, Chang H-C, Chen J-JJ (2012) Modification of platinum microelectrode with molecularly imprinted over-oxidized polypyrrole for dopamine measurement in rat striatum. Sensors Actuators B Chem 171:93–101
Alizadeh N, Eslami MR (2016) Nanostructured conducting molecularly imprinted polypyrrole based quartz crystal microbalance sensor for naproxen determination and its electrochemical impedance study. RSC Advances
Feng W, Liu C, Lu S, Zhang C, Zhu X, Liang Y, Nan J (2014) Electrochemical chiral recognition of tryptophan using a glassy carbon electrode modified with β-cyclodextrin and graphene. Microchim Acta 181(5–6):501–509
Gou H, He J, Mo Z, Wei X, Hu R, Wang Y (2015) An electrochemical chiral sensor for tryptophan enantiomers based on reduced graphene oxide/1, 10-phenanthroline copper (ii) functional composites. RSC Adv 5(74):60638–60645
Tang K, Yi J, Huang K, Zhang G (2009) Biphasic recognition chiral extraction: a novel method for separation of mandelic acid enantiomers. Chirality 21(3):390–395
Martin A, Cocero M (2007) Separation of enantiomers by diastereomeric salt formation and precipitation in supercritical carbon dioxide: application to the resolution of mandelic acid. J Supercrit Fluids 40(1):67–73
Aneja R, Luthra PM, Ahuja S (2010) High-performance liquid chromatography separation of enantiomers of mandelic acid and its analogs on a chiral stationary phase. Chirality 22(5):479–485
Lin J, Hu Q-S, Xu M-H, Pu L (2002) A practical enantioselective fluorescent sensor for mandelic acid. J Am Chem Soc 124(10):2088–2089
Zor E, Patir IH, Bingol H, Ersoz M (2013) An electrochemical biosensor based on human serum albumin/graphene oxide/3-aminopropyltriethoxysilane modified ITO electrode for the enantioselective discrimination of d-and l-tryptophan. Biosens Bioelectron 42:321–325
Xu L, Yang Y, Wang Y, Gao J (2009) Chiral salen Mn (III) complex-based enantioselective potentiometric sensor for l-mandelic acid. Anal Chim Acta 653(2):217–221
Fu Y, Huang T, Chen B, Shen J, Duan X, Zhang J, Li W (2013) Enantioselective resolution of chiral drugs using BSA functionalized magnetic nanoparticles. Sep Purif Technol 107:11–18
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4(8):4806–4814
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7):1558–1565
Han C, Hou X, Zhang H, Guo W, Li H, Jiang L (2011) Enantioselective recognition in biomimetic single artificial nanochannels. J Am Chem Soc 133(20):7644–7647
Ramanavičius A, Ramanavičienė A, Malinauskas A (2006) Electrochemical sensors based on conducting polymer—polypyrrole. Electrochim Acta 51(27):6025–6037
Lisdat F, Schäfer D (2008) The use of electrochemical impedance spectroscopy for biosensing. Anal Bioanal Chem 391(5):1555–1567
Lin M, Han H, Pan D, Zhang H, Su Z (2015) Voltammetric determination of total dissolved iron in coastal waters using a glassy carbon electrode modified with reduced graphene oxide, methylene blue and gold nanoparticles. Microchim Acta 182(3–4):805–813
Moraes FC, Cesarino I, Cesarino V, Mascaro LH, Machado SA (2012) Carbon nanotubes modified with antimony nanoparticles: a novel material for electrochemical sensing. Electrochim Acta 85:560–565
Hsueh C, Brajter-Toth A (1994) Electrochemical preparation and analytical applications of ultrathin overoxidized polypyrrole films. Anal Chem 66(15):2458–2464
Bazzaoui M, Martins L, Bazzaoui E, Martins J (2002) New single-step electrosynthesis process of homogeneous and strongly adherent polypyrrole films on iron electrodes in aqueous medium. Electrochim Acta 47(18):2953–2962
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Zakrzewski V, Montgomery Jr J, Stratmann RE, Burant J (1998) Gaussian 98, revision a. 7; gaussian. Inc, Pittsburgh, PA:12
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38(6):3098
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+ G basis set for first-row elements, Li–F. J Comput Chem 4(3):294–301
Iacob B-C, Bodoki E, Florea A, Bodoki AE, Oprean R (2015) Simultaneous Enantiospecific recognition of several β-blocker enantiomers using molecularly imprinted polymer-based electrochemical sensor. Anal Chem 87(5):2755–2763
Ozkorucuklu SP, Sahin Y, Alsancak G (2008) Voltammetric behaviour of sulfamethoxazole on electropolymerized-molecularly imprinted overoxidized polypyrrole. Sensors 8(12):8463–8478
Palmisano F, Malitesta C, Centonze D, Zambonin P (1995) Correlation between permselectivity and chemical structure of overoxidized polypyrrole membranes used in electroproduced enzyme biosensors. Anal Chem 67(13):2207–2211
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Borazjani, M., Mehdinia, A., Ziaei, E. et al. Enantioselective electrochemical sensor for R-mandelic acid based on a glassy carbon electrode modified with multi-layers of biotin-loaded overoxidized polypyrrole and nanosheets of reduced graphene oxide. Microchim Acta 184, 611–620 (2017). https://doi.org/10.1007/s00604-016-1997-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1997-y