Abstract
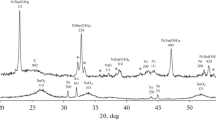
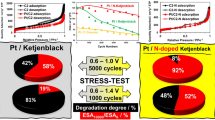
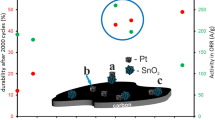
New nanostructured Pt/(SnO2/C)-electrocatalyst (20 wt % Pt) is synthesized via platinum chemical deposited onto composite SnO2/C-support microparticles (4 wt % Sn). The composite support was prepared beforehand using unique method of the tin electrochemical deposition onto disperse carbon black particles. It was shown by X-ray diffraction and transmission electron microscopy that the platinum and tin oxide nanoparticles distributed over the carbon surface are sized 2.4 and 2.9 nm, respectively. Electrochemical measurements showed the obtained catalyst to approach the commercial Pt/C HiSPEC 3000 catalyst (20 wt % Pt) with respect to its mass-activity in the oxygen electroreduction reaction and to be superior thereto as for the electrochemically active surface area, stability in stress test, and activity in methanol electrooxidation reaction. The peculiarities in electrochemical behavior of the synthesized Pt/(SnO2/C)-electrocatalyst can be explained by the SnO2 nanoparticle effect on the platinum nanoparticle nucleation/growth, as well as presence of Pt–SnO2–C triple junction nanostructure at the surface. The Pt/SnO2 contact provides stable platinum-to-support adhesion and asserts bifunctional catalysis mechanism of the methanol electrooxidation. And the Pt/C junctions provide for electron supplying/retraction to or from the platinum nanoparticles.








Similar content being viewed by others
REFERENCES
Debe, M.K., Electrocatalyst approaches and challenges for automotive fuel cells, Nature, 2012, vol. 486, no. 7401, p. 43. https://doi.org/10.1038/nature11115
Yaroslavtsev, A.B., Dobrovolsky, Yu.A., Shaglaeva, N.S., Frolova, L.A., Gerasimova, E.V., and Sanginov, E.A., Nanostructured materials for low-temperature fuel cells, Russ. Chem. Rev., 2012, vol. 81, p. 191.
Liao, H., Qiu, Z., Wan, Q., Wang, Z., Liu, Y., and Yang, N., Universal Electrode Interface for Electrocatalytic Oxidation of Liquid Fuels, ACS Appl. Mater. Interfaces, 2014, vol. 6, no. 20, p. 18055. https://doi.org/10.1021/am504926r
Gong, K., Du, F., Xia, Z., Durstock, M., and Dai, L., Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction, Science, 2009, vol. 323, no. 5915, p. 760. https://doi.org/10.1126/science.1168049
Jin, S., Chen, M., Dong, H., He, B., Lu, H., Su, L., Dai, W., Zhang, Q., and Zhang, X., Stable silver nanoclusters electrochemically deposited on nitrogen-doped graphene as efficient electrocatalyst for oxygen reduction reaction, J. Power Sources, 2015, vol. 274, p. 1173. https://doi.org/10.1016/j.jpowsour.2014.10.098
Liu, S., Zhang, Q., Li, Y., Han, M., Gu, L., Nan, C., Bao, J., and Dai, Z., Five-Fold Twinned Pd2NiAg Nanocrystals with Increased Surface Ni Site Availability to Improve Oxygen Reduction Activity, J. Am. Chem. Soc., 2015, vol. 137, no. 8, p. 2820. https://doi.org/10.1021/ja5129154
Ma, T.Y., Ran, J., Dai, S., Jaroniec, M., and Qiao, S.Z., Phosphorus-Doped Graphitic Carbon Nitrides Grown in situ on Carbon-Fiber Paper: Flexible and Reversible Oxygen Electrodes, Angew. Chem. Int. Ed. 2014, vol. 54, no. 15, p. 4646. https://doi.org/10.1002/anie.201411125
Guterman, V.E., Belenov, S.V., Alekseenko, A.A., Tabachkova, N.Yu., and Volochaev, V.A., The relationship between activity and stability of deposited platinum-carbon electrocatalysts, Russ. J. Electrochem., 2017, vol. 53, p. 531.
Lim, C.S., Wang, L., Chua, C.K., Sofer, Z., Jankovský, O., and Pumera, M., High temperature superconducting materials as bi-functional catalysts for hydrogen evolution and oxygen reduction, J. Mater. Chem. A, 2015, vol. 3, no. 16, p. 8346. https://doi.org/10.1039/c4ta06767c
Qiao, Y. and Li, C.M., Nanostructured catalysts in fuel cells, J. Mater. Chem., 2011, vol. 21, no. 12, p. 4027. https://doi.org/10.1039/c0jm02871a
Hasche, F., Oezaslan, M., and Strasser, P., Activity, stability, and degradation mechanisms of dealloyed PtCu3 and PtCo3 nanoparticle fuel cell catalysts, ChemCatChem., 2011, vol. 3, p. 1805.
Alekseenko, A.A., Belenov, S.V., Volochaev, V.A., Novomlinskiy, I.N., and Guterman, V.E., Cu@Pt/C catalysts: synthesis, structure, activity in oxygen reduction reaction, In Advanced Materials – Techniques, Physics, Mechanics, and Applications, Parinov, I.A., Ed. Heidelberg: Springer Proceedings in Physics, vol. 193, 2017.
Gojković, S., Babić, B., Radmilović, V., and Krstajić, N., 2010, Nb-doped TiO2 as a support of Pt and Pt–Ru anode catalyst for PEMFCs, J. Electroanal. Chem., vol. 639, no. 1–2, p. 161. https://doi.org/10.1016/j.jelechem.2009.12.004
Ho, V.T.T., Pan, C.-J., Rick, J., Su, W.-N., and Hwang, B.-J., Nanostructured Ti0.7Mo0.3O2 Support Enhances Electron Transfer to Pt: High-Performance Catalyst for Oxygen Reduction Reaction, J. Am. Chem. Soc., 2011, vol. 133, no. 30, p. 11716. https://doi.org/10.1021/ja2039562
Ignaszak, A., Song, C., Zhu, W., Wang, Y.-J., Zhang, J., Bauer, A., Baker, R., Neburchilov, V., Campbell, S., and Ye, S., Carbon–Nb0.07Ti0.93O2 composite supported Pt–Pd electrocatalysts for PEM fuel cell oxygen reduction reaction, Electrochim, Acta, 2012, vol. 75, p. 220. https://doi.org/10.1016/j.electacta.2012.04.111
Jukk, K., Kongi, N., Tarre, A., Rosental, A., Treshchalov, A., Kozlova, J., Ritslaid, P., Matisen, L., Sammelselg, V., and Tammeveski, K., Electrochemical oxygen reduction behaviour of platinum nanoparticles supported on multi-walled carbon nanotube/titanium dioxide composites, J. Electroanal. Chem., 2014, vol. 735, p. 68. https://doi.org/10.1016/j.jelechem.2014.10.008
Tiido, K., Alexeyeva, N., Couillard, M., Bock, C., Macdougall, B.R., and Tammeveski, K., Graphene–TiO2 composite supported Pt electrocatalyst for oxygen reduction reaction, Electrochim. Acta, 2013, vol. 107, p. 509. https://doi.org/10.1016/j.electacta.2013.05.155
Cui, X., Shi, J., Chen, H., Zhang, L., Guo, L., Gao, J., and Li, J., Platinum/Mesoporous WO3 as a Carbon-Free Electrocatalyst with Enhanced Electrochemical Activity for Methanol Oxidation, J. Phys. Chem. B, 2008, vol. 112, no. 38, p. 12024. https://doi.org/10.1021/jp803565k
Ioroi, T., Siroma, Z., Fujiwara, N., Yamazaki, S.-I., and Yasuda, K., Sub-stoichiometric titanium oxide-supported platinum electrocatalyst for polymer electrolyte fuel cells, Electrochem. Commun., 2005, vol. 7, no. 2, p. 183. https://doi.org/10.1016/j.elecom.2004.12.007
Lei, B., Xue, J., Jin, D., Ni, S., and Sun, H., Fabrication, annealing, and electrocatalytic properties of platinum nanoparticles supported on self-organized TiO2 nanotubes, Rare Metals, 2008, vol. 27, no. 5, p. 445. https://doi.org/10.1016/s1001-0521(08)60160-6
Shim, J., Lee, C.-R., Lee, H.-K., Lee, J.-S., and Cairns, E.J., Electrochemical characteristics of Pt–WO3/C and Pt–TiO2/C electrocatalysts in a polymer electrolyte fuel cell, J. Power Sources, 2001, vol. 102, no. 1–2, p. 172. https://doi.org/10.1016/s0378-7753(01)00817-5
Vayssilov, G.N., Lykhach, Y., Migani, A., Staudt, T., Petrova, G.P., Tsud, N., Skála, T., Bruix, A., Illas, F., Prince, K.C., Matolın, V., Neyman, K.M., and Libuda, J., Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles, Nature Materials, 2011, vol. 10, no. 4, p. 310. https://doi.org/10.1038/nmat2976
Wang, M., Guo, D.-J., and Li, H.-L., High activity of novel Pd/TiO2 nanotube catalysts for methanol electro-oxidation, J. Solid State Chem., 2005, vol. 178, no. 6, p. 1996. https://doi.org/10.1016/j.jssc.2005.04.006
Dou, M., Hou, M., Liang, D., Lu, W., Shao, Z., and Yi, B., SnO2 nanocluster supported Pt catalyst with high stability for proton exchange membrane fuel cells, Electrochim, Acta, 2013, vol. 92, p. 468. https://doi.org/10.1016/j.electacta.2013.01.070
Nakada, M., Ishihara, A., Mitsushima, S., Kamiya, N., and Ota, K.-I., Effect of Tin Oxides on Oxide Formation and Reduction of Platinum Particles, Electrochem. Solid State Lett., 2007, vol. 10, no. 1. https://doi.org/10.1149/1.2382263
Tripković, D., Stevanović, S., Gavrilović, A., Rogan, J., Lačnjevac, U., Kravić, T., and Jovanović, V.M., The Role of SnO2 on Electrocatalytic Activity of PtSn Catalysts, Electrocatalysis, 2017, vol. 9, no. 1, p. 76. https://doi.org/10.1007/s12678-017-0424-4
Frolova, L.A., Dobrovolsky, Y.A., and Bukun, N.G., Oxide supported platinum electrocatalysts for hydrogen and alcohol fuel cells, Russ. J. Electrochem., 2011, vol. 47, no. 6, p. 697. https://doi.org/10.1134/s1023193511060024
Antoniassi, R., Silva, J., Neto, A.O., and Spinacé, E., Synthesis of Pt SnO2/C electrocatalysts containing Pt nanoparticles with preferential (100) orientation for direct ethanol fuel cell, Appl. Catal. B Environ., 2017, vol. 218, p. 91. https://doi.org/10.1016/j.apcatb.2017.06.031
Jia, J., Wang, H., Ji, S., Yang, H., Li, X., and Wang, R., SnO2-embedded worm-like carbon nanofibers supported Pt nanoparticles for oxygen reduction reaction, Electrochim. Acta, 2014, vol. 141, p. 13. https://doi.org/10.1016/j.electacta.2014.07.020
Huang, M., Zhang, J., Wu, C., and Guan, L., Pt nanoparticles Densely Coated on SnO2-Covered Multiwalled Carbon Nanotubes with Excellent Electrocatalytic Activity and Stability for Methanol Oxidation, ACS Appl. Mater. Interfaces, 2017, vol. 9, no. 32, p. 26921. https://doi.org/10.1021/acsami.7b07866
Zhang, K., Feng, C., He, B., Dong, H., Dai, W., Lu, H., and Zhang, X., An advanced electrocatalyst of Pt decorated SnO2/C nanofibers for oxygen reduction reaction, J. Electroanal. Chem., 2016, vol. 781, p. 198. https://doi.org/10.1016/j.jelechem.2016.11.002
Zhang, N., Zhang, S., Du, C., Wang, Z., Shao, Y., Kong, F., Lin, Y., and Yin, G., Pt/Tin Oxide/Carbon Nanocomposites as Promising Oxygen Reduction Electrocatalyst with Improved Stability and Activity, Electrochim. Acta, 2014, vol. 117, p. 413. https://doi.org/10.1016/j.electacta.2013.11.139
Parrondo, J., Mijangos, F., and Rambabu, B., Platinum/tin oxide/carbon cathode catalyst for high temperature PEM fuel cell, J. Power Sources, 2010, vol. 195, no. 13, p. 3977. https://doi.org/10.1016/j.jpowsour.2010.01.027
Kuriganova, A.B., Leontyeva, D.V., Ivanov, S., Bund, A., and Smirnova, N.V., Electrochemical dispersion technique for preparation of hybrid MOx–C supports and Pt/MOx–C electrocatalysts for low-temperature fuel cells, J. Appl. Electrochem., 2016, vol. 46, no. 12, p. 1245. https://doi.org/10.1007/s10800-016-1006-5
Kuriganova, A.B. and Smirnova, N.V., Pt/SnOx–C composite material for electrocatalysis, Mendeleev Commun., 2014, vol. 24, no. 6, p. 351. https://doi.org/10.1016/j.mencom.2014.11.013
Alekseenko, A.A., Guterman, V.E., Volochaev, V.A., and Belenov, S.V., Effect of wet synthesis conditions on the microstructure and active surface area of Pt/C catalysts, Inorg. Mater., 2015, vol. 51, no. 12, p. 1258. https://doi.org/10.1134/s0020168515120018
Guterman, V.E., Lastovina, T.A., Belenov, S.V., Tabachkova, N.Y., Vlasenko, V.G., Khodos, I.I., and Balakshina, E.N., PtM/C (M = Ni, Cu, or Ag) electrocatalysts: effects of alloying components on morphology and electrochemically active surface areas, J. Solid State Electrochem., 2013, vol. 18, no. 5, p. 1307. https://doi.org/10.1007/s10008-013-2314-x
Vliet, D.V.D., Strmcnik, D.S., Wang, C., Stamenkovic, V.R., Markovic, N.M., and Koper, M.T., On the importance of correcting for the uncompensated Ohmic resistance in model experiments of the Oxygen Reduction Reaction, J. Electroanal. Chem., 2010, vol. 647, no. 1, p. 29. https://doi.org/10.1016/j.jelechem.2010.05.016
Shinozaki, K., Zack, J.W., Pylypenko, S., Pivovar, B.S., and Kocha, S.S., Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique, J. Electrochem. Soc., 2015, vol. 162, no. 12. https://doi.org/10.1149/2.0551512jes
Jeon, M.K., Zhang, Y., and Mcginn, P.J., A comparative study of PtCo, PtCr, and PtCoCr catalysts for oxygen electro-reduction reaction, Electrochim. Acta, 2010, vol. 55, no. 19, p. 5318. https://doi.org/10.1016/j.electacta.2010.04.056
Fabbri, E., Rabis, A., Kötz, R., and Schmidt, T.J., Pt nanoparticles supported on Sb-doped SnO2 porous structures: developments and issues, Phys. Chem. Chem. Phys., 2014, vol. 16, no. 27, p. 13672. https://doi.org/10.1039/c4cp00238e
Wang, J., Yin, G., Shao, Y., Zhang, S., Wang, Z., and Gao, Y., Effect of carbon black support corrosion on the durability of Pt/C catalyst, J. Power Sources, 2007, vol. 171, no. 2, p. 331. https://doi.org/10.1016/j.jpowsour.2007.06.084
Thompsett, D., Catalysts for the proton exchange membrane fuel cell, in: Handbook of Fuel Cells. Fundamentals, Technology and Applications, Vielstich, W., Lamm, A., and Gasteiger, H.A., Eds., New York: Wiley, 2003, p. 6–23.
Park, Yu-Ch., Kakinuma, K., Uchida, M., Uchida, H., and Watanabe, M., Deleterious effects of interim cyclic voltammetry on Pt/carbon black catalyst degradation during start-up/shutdown cycling evaluation, Electrochim. Acta, 2014, vol. 123, p. 84.
Eris, S., Daşdelen, Z., and Sen, F., Enhanced electrocatalytic activity and stability of monodisperse Pt nanocomposites for direct methanol fuel cells, J. Colloid Interface Sci., 2018, vol. 513, p. 767. https://doi.org/10.1016/j.jcis.2017.11.085
Funding
This study is performed within the framework of the state target of the Ministry of education and sciences of RF (topic no. 13.3005.2017/4.6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
Rights and permissions
About this article
Cite this article
Novomlinskiy, I.N., Guterman, V.E., Danilenko, M.V. et al. Platinum Electrocatalysts Deposited onto Composite Carbon Black–Metal Oxide Support. Russ J Electrochem 55, 690–700 (2019). https://doi.org/10.1134/S1023193519070097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193519070097