Abstract
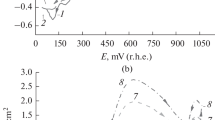
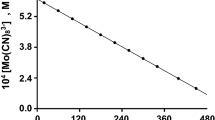
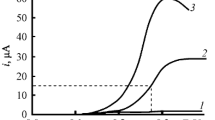
The model reagents substrates, 1-butanol and 1-nonanol are used to study the general kinetic regularities of indirect electrocatalytic oxidation of aliphatic alcohols on conventional (Pb/PbO2) and catalytically active (oxide–hydroxide–nickel) electrodes to the corresponding carboxylic acids (butyric and pelargonic) at the participation of active oxygen forms (AOF) generated in situ from O2, H2O2, and H2O. It is found that the kinetics of the alcohol oxidation reaction correspond to the pseudo first order when the studied AOF generation schemes are used. The effect of the nature of electrode materials on the kinetic regularities of oxidation of aliphatic alcohols to the corresponding carboxylic acids is established for the aspects pointing to the presence of different active forms of bound oxygen determining the possible reaction routes. On the basis of the obtained results, the main possible oxidation routes with the participation of active oxygen generated in situ are considered.
Similar content being viewed by others
References
Budnikova, Yu.G., Ross. Khim. Zh., 2005, vol. 49, no. 5, p. 81.
Schäfer, H.J., C. R. Chim., 2011, vol. 14, p. 745.
Frontana-Uribe, B.A., Little, R.D., Ibanez, J.G., Palma, A., and Vasquez-Medrano, R., Green Chem., 2010, vol. 12, p. 2099.
Vysotskaya, N.A., Usp. Khim., 1973, vol. 47, p. 1843.
Sychev, A.Ya., Travin, S.O., Duka, G.G., and Skurlatov, Yu.I., Kataliticheskie reaktsii i okhrana okruzhayushchei sredy (Catalytic Reactions and Protection of Environment), Chisinau: Shtiintsa, 1983.
Brillas, E. and Martinez-Huitle, C.A., Synthetic Diamond Films: Preparation, Chemistry, Characterization and Applications, Hoboken, New Jersey, 2011.
Kornienko, V.L., Khim. Interesakh Ustoich. Razvit., 2002, vol. 10, no. 4, p. 391.
Kolyagin, G.A. and Kornienko, V.L., Khim. Interesakh Ustoich. Razvit., 2000, vol. 8, no. 6, p. 803.
Kapalka, A., Foti, G., and Comninellis, Ch., Electrochim. Acta, 2009, vol. 54, p. 2018.
Buxton, G.V., Clive, L., Greenstok, C.L., Helman, W.Ph., and Ross, A.R., J. Phys. Chem. Ref. Data, 1988, vol. 17, p. 513.
Brillas, E., Sires, I., and Oturan, M.A., Chem. Rev., 2009, vol. 109, p. 6570.
Pillai, U.R. and Sahle-Demessie, E., Appl. Catal., A, 2003, vol. 245, p. 103.
Chaenko, N.V., Kornienko, G.V., Kosheleva, A.M., Maksimov, N.G., and Kornienko, V.L., Russ. J. Electrochem., 2011, vol. 47, p. 1146.
Chen, Y.-L. and Chou, T.-C., J. Applied Electrochem., 1996, vol. 26, p. 543.
Remorov, B.S., Avrutskaya, I.A., and Fioshin, M.Ya., Elektrokhimiya, 1981, vol. 17, p. 1547.
Fleischmann, M., Korinek, K., and Pletcher, D., J. Chem. Soc., Perkin Trans. 2, 1972, p. 1396.
Kishioka, S., Ohki, S., Ohsaka, T., and Tokuda, K., J. Electroanal. Chem. Interfacial Electrochem., 1998, vol. 452, p. 179.
Chen, Y.-L. and Chou, T.-C., Ind. Eng. Chem. Res., 1996, vol. 35, p. 2172.
Schäfer, H.J., Top. Curr. Chem., 1987, vol. 142, p. 102.
Lyalin, B.V. and Petrosyan, V.A., Russ. J. Electrochem., 2010, vol. 46, p. 1199.
Lyalin, B.V. and Petrosyan, V.A., Russ. J. Electrochem., 2011, vol. 47, p. 1236.
Ogibin, Yu.N., Elinson, N.M., and Nikishin, G.I., Russ. Chem. Rev., 2009, vol. 78, p. 89.
Kosheleva, A.M., Chaenko, N.V., Kornienko, G.V., Vlasenko, V.I., and Kornienko, V.L., Russ. J. Electrochem., 2013, vol. 49, p. 96.
Liu, J., Ye, J., Xu, C., Jiang, S.P., and Tong, Y., Electrochem. Commun., 2007, vol. 9, p. 2334.
Tarasevich, M.R., Karichev, Z.R., Bogdanovskaya, V.A., Lubnin, E.N., and Kapustin, A.V., Electrochem. Commun., 2005, vol. 7, p. 141.
Tarasevich, M.R. and Kuzov, A.V., Al’tern. Energ. Ekol., 2010, no. 7, p. 86.
Chaenko, N.V., Kornienko, V.L., Avrutskaya, I.A., and Fioshin, M.Ya., Zh. Prikl. Khim., 1987, vol. 60, p. 1339.
Kaulen, I. and Schäfer, H., Tetrahedron, 1982, vol. 38, p. 3299.
Chaenko, N.V., Kornienko, G.V., Sokolenko, V.A., and Kornienko, V.L., Russ. J. Appl. Chem., 2014, vol. 87, p. 451.
Dzhafarov, E.A., Elektroosazhdenie, svoistva i primenenie dvuokisi svintsa (Electrodeposition, Properties, and Application of Lead Dioxide), Baku: Az. SSR, 1967.
Kornienko, G.V., Chaenko, N.V., and Kornienko, V.L., Khim. Interesakh Ustoich. Razvit., 2006, vol. 14, no. 1, p. 23.
Gerasimov, Ya.I., Kurs fizicheskoi khimii (Course in Physical Chemistry), Moscow: Khimiya, 1973, vol. 2.
Giamello, E., Rumori, P., Fubini, B., and Paganini, M.C., Appl. Magn. Reson., 1996, vol. 1, p. 173.
Imamoto, M. and Lunsford, J.H., J. Phys. Chem., 1980, vol. 84, p. 3079.
Bielski, B.H.J., Cabelli, D.E., and Arudi, R.L., J. Phys. Chem. Ref. Data, 1985, vol. 14, p. 1041.
Simond, O., Schaller, V., and Comninellis, Ch., Electrochim. Acta, 1997, vol. 42, p. 2009.
Simic, M., Neta, P., and Hayon, E., J. Phys. Chem., 1969, vol. 73, p. 3794.
Bianchini, C. and Shen, P.K., Chem. Rev., 2009, vol. 109, p. 4183.
Miyoshi, A., Matsui, H., and Washida, N., J. Phys. Chem., 1990, vol. 94, p. 3016.
Remorov, B.S., Avrutskaya, I.A., and Fioshin, M.Ya., Elektrokhimiya, 1981, vol. 17, p. 743.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 18th AllRussian Conference on Organic Electrochemistry, Tambov, September 15–19, 2014.
Original Russian Text © A.M. Kosheleva, N.G. Maksimov, G.V. Kornienko, V.L. Kornienko, 2015, published in Elektrokhimiya, 2015, Vol. 51, No. 11, pp. 1216–1222.
Rights and permissions
About this article
Cite this article
Kosheleva, A.M., Maksimov, N.G., Kornienko, G.V. et al. Studies of kinetics of indirect in situ electrocatalytic oxidation of aliphatic alcohols to carboxylic acids by active forms of oxygen. Russ J Electrochem 51, 1079–1085 (2015). https://doi.org/10.1134/S1023193515110075
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193515110075