Abstract
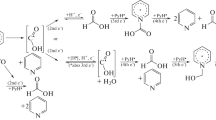
The electrooxidation of ethanol (C2H5OH) is studied on the surface of platinum (Pt) electrode in 1.0 М aqueous solutions of methanesulfonic acid (CH3SO3H). It is found that the complete displacement of adsorbed hydrogen from the Pt surface in the potential region of 0.03–0.4 V (r.h.e.) occurs at the ethanol concentration 2.0 М C2H5OH. In the anodic scanning region, three ethanol oxidation waves are observed in the potential regions E: 0.8–1.1, 1.15–1.45, and 1.5–1.8 V (r.h.e.). The analysis of products of preparative electrolysis in the aforementioned potential regions carried out by the methods of molecular (UV, near IR-Fourier and Raman) spectroscopy has shown that the first wave of ethanol oxidation corresponds to the formation of acetaldehyde, the second wave corresponds to acetic acid, and the third wave is associated with the formation of carbon dioxide (СO2). In the reverse cathodic scan, the anodic wave with the peak at 0.55 V (r.h.e.) appears which is associated with the direct oxidation of ethanol to СO2. It is assumed that the mechanism of ethanol electrooxidation on Pt in 1.0 М СH3SO3H is analogous to that realized in sulfuric acid solutions.






Similar content being viewed by others
REFERENCES
Palden, T., Onghena, B., Regadío, M., and Binnemans, K., Methanesulfonic acid: a sustainable acidic solvent for recovering metals from jarosite residue of the zinc industry, Green Chem., 2019, vol. 21, p. 5394. https://doi.org/10.1039/c9gc02238d
Walsh, F.C. and Ponce de León, C., Versatile electrochemical coatings and surface layers from aqueous methanesulfonic acid, Surf. Coat. Technol., 2014, vol. 259, p. 676. https://doi.org/10.1016/j.surfcoat.2014.10.010
Kim, G., Kim, Y., Yim, T., and Kwon, K., Effects of methanesulfonic acid on electrolyte for vanadium redox flow batteries, J. Ind. Eng. Chem., 2021, vol. 99, p. 326. https://doi.org/10.1016/j.jiec.2021.04.043
Krishna, M., Wallis, L.P.J., Wills, R.G.A., Hall, D., and Shah, A.A., Measurement of key electrolyte properties for improved performance of the soluble lead flow battery, Int. J. Hydrogen Energy, 2017, vol. 42(29), p. 18491. https://doi.org/10.1016/j.ijhydene.2017.05.0
Vijayalekshmi, V. and Khastgir, D., Eco-friendly methanesulfonic acid and sodium salt of dodecylbenzene sulfonic acid doped cross-linked chitosan based green polymer electrolyte membranes for fuel cell applications, J. Membr. Sci., 2017, vol. 523, p. 45. https://doi.org/10.1016/j.memsci.2016.09.058
Akhmedov, M.A., Khidirov, S.S., and Khibiev, K.S., Modification of cellulose in the solution of methanesulfonic acid. Russ. Chem. Bull., 2021, no. 2(70), p. 412. https://doi.org/10.1007/s11172-021-3101-y
Akhmedov, M.A. and Khidirov, Sh.Sh., Anodic processes at smooth platinum electrode in concentrated solution of methanesulfonic acid, Russ. J. Electrochem., 2019, vol. 55, p. 579. https://doi.org/10.1134/S1023193519060028
Akhmedov, M.A. and Khidirov, Sh.Sh., Voltammetric determination of the composition and properties of methanesulfonic acid, J. Struct. Chem., 2014, vol. 55, no. 6, p. 1148. https://doi.org/10.1134/S0022476614060249
Sandoval, A.P., Suárez-Herrera, M.F., Climent, V., and Feliu, J.M., Interaction of water with methanesulfonic acid on Pt single crystal electrodes, Electrochem. Comm., 2015, vol. 50, p. 47. https://doi.org/10.1016/j.elecom.2014.11.007
Trasatti, S. and Petrii, O.A., Real surface area measurements in electrochemistry, J. Electroanal. Chem., 1992, vol. 327, p. 353. https://doi.org/10.1016/0022-0728(92)80162-w
Bagotsky, V.S. and Vasiliev, Yu.B., in Uspekhi elektrokhimii organicheskikh soedinenii (Successes in Electrochemistry of Organic Compounds), Moscow: Nauka, 1966, p. 40.
Damaskin, B.B. and Petrii, O.A., Vvedenie v elektrokhimicheskuyu kinetiku (Introduction to Electrochemical Kinetics), Moscow: Vyssh. Shkola, 1983.
Scholz, F., Electro-analytical Methods. Guide to Experiments and Applications, Berlin: Springer, 2002.
Akhmedov, M.A., Ibragimova, K.O., and Khidirov, Sh.Sh., Comparative evaluation of dimethylsulfoxide and dimethylsulfone adsorption on a smooth platinum electrode in acidic environment, Russ. J. Electrochem., 2020, vol. 56, p. 396. https://doi.org/10.1134/S1023193520040023
Pentin, Yu.A. and Kuramshina, G.M., Osnovy molekulyarnoi spektroskopii (The Fundamentals of Molecular Spectroscopy), Moscow: Mir, 2008.
Burikov, S.A., Dolenko, S., Dolenko, T., Patsaeva Svetlana, and Yuzhakov, V., Decomposition of water Raman stretching band with a combination of optimization methods, Mol. Phys., 2010, vol. 108, no. 6, p. 739. https://doi.org/10.1080/00268970903567288
Harris, K.R., Newitt, P.J., and Derlacki, Z.J., Alcohol tracer diffusion, density, NMR and FTIR studies of aqueous ethanol and 2,2,2-trifluoroethanol solutions at 25°C, J. Chem. Soc. Faraday Trans, 1998, vol. 94, no. 14, p. 1963. https://doi.org/10.1039/a802567c
Dolenko, T.A., Burikov, S.A., Dolenko, S.A., Efitorov, A.O., Plastinin, I.V., Yuzhakov, V.I., and Patsaeva, S.V., Raman spectroscopy of water–ethanol solutions: the estimation of hydrogen bonding energy and the appearance of clathrate-like structures in solutions, J. Phys. Chem. A, 2015, vol. 119, no. 44, p.10806. https://doi.org/10.1021/acs.jpca.5b06678
Kuzov, A.V., Adsorption and electrooxidation of ethanol on platinum-containing catalysts in acidic media, Al’tern. Energ. Ekol., 2010, no. 5(85), p. 126.
Podlovchenko, B.I., On the processes occurring during the introduction of a platinized platinum electrode into solutions of C2H5OH, H–C3H7OH and H–C4H9OH, Elektrokhimiya, 1965, vol. 1, p. 101.
Tarasevich, M.R. and Korchagin, O.V., Electrocatalysis and pH (review), Russ. J. Electrochem., 2013, vol. 49, p. 676. https://doi.org/10.1134/S102319351307015X
Danilov, A.I., Molodkina, E. B., and Polukarov, Yu.M., Surface and subsurface oxygen on platinum. Solution 0.5 M H2SO4, Russ. J. Electrochem., 2004, vol. 40, p. 667.
Tarasevich, M.R., Korchagin, O.V., and Kuzov, A.V., Electrocatalysis of anodic oxidation of ethanol, Russ. Chem. Rev., 2013, no. 11(82), p. 1047. https://doi.org/10.1070/RC2013v082n11ABEH004276
Podlovchenko, B.I., Petrii, O.A., Frumkin, A.N., and Hira Lal, Behavior of a platinized platinum electrode in solutions of alcohols containing more than one carbon atom, aldehydes and formic acid, J. Electroanal. Chem., 1966, no. 1(11), p. 12. https://doi.org/10.1016/0022-0728(66)80053-0
Suib, S.L., New and Future Developments in Catalysis Batteries, Hydrogen Storage and Fuel Cells, Amsterdam: Elsevier, 2013.
Yaqoob, L., Noor, T., and Iqbal, N., A comprehensive and critical review of the recent progress in electrocatalysts for the ethanol oxidation reaction, RSC Adv., 2021, no. 11(27), p. 16768. https://doi.org/10.1039/D1RA01841H
Izotova, V.V., Tyurin, Yu.M., and Volodin, G.F., Influence of the composition of the solution on the limiting filling of the Pt anode with oxides, Elektrokhimiya, 1970, vol. 6, p. 1186.
Tyurin, Yu.M., Volodin, G.F., and Battalova, Yu.V., Simulation of cathodic CVA curves based on data on the CVA kinetics of oxygen layer reduction, Elektrokhimiya, 1981, vol. 17, p. 241.
Hommond, J.S. and Winograd, N., XPS spectroscopic study of potentiostatic and galvanostatic oxidation of Pt electrodes in H2SO4 and HClO4, J. Electroanal. Chem., 1977, vol. 78, p. 55. https://doi.org/10.1016/S0022-0728(77)80422-1
Yakovleva, A.A., Bairamov, R.K., and Kirsanova, E.V., Study of the adsorption of cesium cations on platinum at high anodic potentials, Elektrokhimiya, 1976, vol. 12, p. 1317.
Tyurin, Yu.M. and Volodin, G.F., Influence of solution composition on the limiting filling of the Pt-anode with oxides, Elektrokhimiya, 1970, vol. 6, p. 1186.
Kazarinov, V.E. and Girina, G.P., Study of the structure of the double electric layer on platinum in the presence of acetate ions, Elektrokhimiya, 1967, vol. 3, p. 107.
Gavrilova, N.N. and Nazarov, V.V., Analiz poristoi struktury na osnove adsorbtsionnykh dannykh (Analysis of Porous Structures Based on Adsorption Data) Moscow: Mendeleev Inst., 2015.
Ng, K.C., Burhan, M., Shahzad, M.W., and Ismail, A.B., A universal isotherm model to capture adsorption uptake and energy distribution of porous heterogeneous surface, Sci. Rep., 2017, vol. 7(1), p. 10634. https://doi.org/10.1038/s41598-017-11156-6
Alavi, S., Ohmura, R., and Ripmeester, J.A., A molecular dynamics study of ethanol–water hydrogen bonding in binary structure I clathrate hydrate with CO2, Chem. Phys., 2011, vol. 134(5), p. 054702. https://doi.org/10.1063/1.3548868
ACKNOWLEDGMENTS
This study was carried out with the use of equipment of the Centers of Collective Use at the Dagestan State University and the Dagestan Federal Research Center of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by T. Safonova
Published based on the materials of the 15th International Conference “Fundamental Problems of Solid State Ionics,” Chernogolovka, November 30–December 07, 2020.
Supplementary Information
Rights and permissions
About this article
Cite this article
Akhmedov, M.A., Khidirov, S.S. Electrocatalytic Oxidation of Ethanol on the Platinum Electrode in Solution of Methanesulfonic Acid. Russ J Electrochem 58, 482–489 (2022). https://doi.org/10.1134/S1023193522060039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193522060039