Abstract
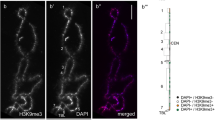
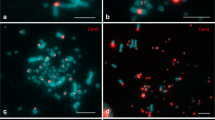
Changes in functional gene activity not affecting primary DNA structure can be inherited by a cell and in total represent epigenetic mechanisms of the genome expression regulation. Epigenetic genome modifications include DNA methylation, histone modifications, and binding of the proteins and noncoding RNA. The distribution of heterochromatin markers (such as methylated cytosine, HP1 heterochromatin protein, and modifications of H3, H4, and H2A histones) was analyzed by example of lampbrush chromosomes typical for growing bird oocytes. On the lampbrush chromosomes of domesticated chicken (Gallus gallus domesticus), Japanese quail (Coturnix japonica), and chaffinch (Fringilla coelebs), methylated cytosine was mainly detected in chromomeres, as well as in unextended regions of lateral loop axes. The largest accumulation of methylated cytosine was demonstrated by chromosome W and dense chromomeres of macro- and microchromosomes. The HP1β protein distribution in the lampbrush chromosomes corresponded in general to the distribution of methylated cytosine. Chromomeres of chromosome W as well as centromeric and terminal chromomeres of macro- and microchromosomes accumulated the highest amount of the HP1β protein as compared with the remaining chromomeres. The character of distribution of the H3K9 me3 and H3K27 me3 histone modifications, as well as phosphorylated H4 and H2A histones, was different. Phosphorylated H4 and H2A histones were distributed proportionally to the staining by DNA specific dyes and were accumulated in all chromosomes, while trimethylated H3K9 histone was enriched in the regions of constitutive heterochromatin. We assume that the HP1β heterochromatin protein is involved in compaction and transcriptional inactivation of the chromatin in chromomeres of the lampbrush chromosomes.
Similar content being viewed by others
References
Peterson, C.L. and Laniel, M.A., Histones and histone modifications, Curr. Biol., 2004, vol. 14, no. 14, pp. 546–551. doi 10.1016/j.cub.2004.07.007
Gaginskaya, E.R., Lampbrush chromosomes from amphibian oocytes, Tsitologiya, 1989, vol. 31, no. 11, pp. 1267–1291.
Callan, H.G., Lampbrush Chromosomes, Berlin: Springer-Verlag, 1986.
Morgan, G.T., Lampbrush chromosomes and associated bodies: new insights into principles of nuclear structure and function, Chromosome Res., 2002, vol. 10, pp. 177–200.
Gaginskaya, E., Kulikova, T., and Krasikova, A., Avian lampbrush chromosomes: a powerful tool for exploration of genome expression, Cytogenet. Genome Res., 2009, vol. 124, pp. 251–267. doi 10.1159/000218130
Kropotova, E.V. and Gaginskaya, E.R., Lampbrush chromosomes from Japanese quail oocytes: light and electron microscopy data, Tsitologiya, 1984, vol. 26, pp. 1006–1015.
Solovei, I., Gaginskaya, E., Hutchison, N., and Macgregor, H., Avian sex chromosomes in the lampbrush form: the ZW lampbrush bivalents from six species of bird, Chromosome Res., 1993, vol. 1, pp. 153–166.
Solovei, I.V., Gaginskaya, E.R., and Macgregor, H.C., The arrangement and transcription of telomere DNA sequences at the ends of lampbrush chromosomes of birds, Chromosome Res., 1994, vol. 2, pp. 460–470.
Sumner, A.T., Chromosomes Organization and Function, Blackwell, 2003.
Angelier, N., Bonnanfant-Jais, M.L., Moreau, N., et al., DNA methylation and RNA transcriptional activity in amphibian lampbrush chromsomes, Chromosoma, 1986, vol. 94, pp. 169–182. doi 10.1007/BF00288491
Morgan, G.T., Jones, P., and Bellini, M., Association of modified cytosines and the methylated DNA-binding protein MeCP2 with distinctive structural domains of lampbrush chromatin, Chromosome Res., 2012, vol. 20, pp. 925–942. doi 10.1007/s10577-012-9324-x
Jones, P.L., Veenstra, G.J., Wade, P.A., et al., Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription, Nat. Genet., 1998, vol. 19, no. 2, pp. 187–191. doi 10.1038/561
Choo, K.H., Centromerization, Trends Cell Biol., 2000, vol. 10, pp. 182–188.
Choo, K.H., Domain organization at the centromere and neocentromere, Dev. Cell, 2001, vol. 1, no. 2, pp. 165–177.
Solovei, I.V., Joffe, B.I., Gaginskaya, E.R., and Macgregor, H.C., Transcription on lampbrush chromosomes of a centromerically localized highly repeated DNA in pigeon (Columba) relates to sequence arrangement, Chromosome Res., 1996, vol. 4, pp. 588–603.
Saifitdinova, A., Derjusheva, S., Krasikova, A., and Gaginskaya, E., Lampbrush chromosomes of the chaffinch (Fringilla coelebs L.), Chromosome Res., 2003, vol. 11, pp. 99–113.
Krasikova, A., Deryusheva, S., Galkina, S., et al., On the positions of centromeres in chicken lampbrush chromosomes, Chromosome Res., 2006, vol. 14, no. 7, pp. 777–789. doi 10.1007/s10577-006-1085-y
Krasikova, A., Daks, A., Zlotina, A., and Gaginskaya, E., Polymorphic heterochromatic segments in Japanese quail microchromosomes, Cytogenet. Genome Res., 2009, vol. 126, pp. 148–155. doi 10.1159/000245914
Stewart, M.D., Sommerville, J., and Wong, J., Dynamic regulation of histone modifications in Xenopus oocytes through histone exchange, Mol. Cell Biol., 2006, vol. 26, no. 18, pp. 6890–6901. doi 10.1128/MCB.00948-06
Zlotina, A., Kulikova, T., Kosyakova, N., et al., Microdissection of lampbrush chromosomes as an approach for generation of locus-specific FISH-probes and samples for high-throughput sequencing, BMC Genomics, 2016, vol. 17, p. 126. doi 10.1186/s12864-016-2437-4
Barber, C.M., Turner, F.B., Wang, Y., et al., The enhancement of histone H4 and H2A serine 1 phosphorylation during mitosis and S-phase is evolutionarily conserved, Chromosoma, 2004, vol. 112, no. 7, pp. 360–371. doi 10.1007/s00412-004-0281-9
Zhang, Y., Griffin, K., Mondal, N., and Parvin, J.D., Phosphorylation of histone H2A inhibits transcription on chromatin templates, J. Biol. Chem., 2004, vol. 279, pp. 21866–21872. doi 10.1074/jbc.M400099200
Hock, R., Moorman, A., Fischer, D., and Scheer, U., Absence of somatic histone H1 in oocytes and preblastula embryos of Xenopus laevis, Dev. Biol., 1993, vol. 158, no. 2, pp. 510–522. doi 10.1006/dbio.1993.1209
Maison, C. and Almouzni, G., HP1 and the dynamics of heterochromatin maintenance, Nat. Rev. Mol. Cell Biol., 2004, vol. 5, no. 4, pp. 296–304. doi 10.1038/nrm1355
Kimmins, S. and Sassone-Corsi, P., Chromatin remodelling and epigenetic features of germ cells, Nature, 2005, vol. 434, no. 7033, pp. 583–589. doi 10.1038/nature03368
Henikoff, S., Heterochromatin function in complex genomes, Biochim. Biophys. Acta, 2000, vol. 1470, pp. O1–O8.
Redi, C.A., Garagna, S., Zacharias, H., et al., The other chromatin, Chromosoma, 2001, vol. 110, pp. 136–147.
Hori, T., Suzuki, Y., Solovei, I., et al., Characterization of DNA sequences constituting the terminal heterochromatin of the chicken Z chromosome, Chromosome Res., 1996, vol. 4, no. 6, pp. 411–426.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Krasikova, T.V. Kulikova, 2017, published in Genetika, 2017, Vol. 53, No. 9, pp. 1077–1085.
Rights and permissions
About this article
Cite this article
Krasikova, A.V., Kulikova, T.V. Distribution of heterochromatin markers in lampbrush chromosomes in birds. Russ J Genet 53, 1022–1029 (2017). https://doi.org/10.1134/S1022795417090071
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795417090071