Abstract
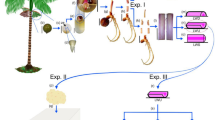
An embryogenic culture of poppy Papaver rupifragum Boiss. & Reut., which was initiated on the roots of seedlings grown from seeds on MS medium with the addition of 1 mg/L IBA, was obtained for the first time. Subsequent maintenance of the embryogenic culture was carried out on a hormone-free MS medium. Long-term cultivation and preservation of the embryogenic capacity of the culture (more than 5 years) was supported by cycles of adventitious embryoidogenesis, including the formation of callus on preexisting embryoids and the induction of new embryoids from their subsurface cells. In this regard, the resulting P. rupifragum culture can be considered as a differentiated culture in which the callus stage is an intermediate stage of development. It has been established that the surface of newly formed embryoids is covered with a surface network of extracellular matrix formed by polysaccharides, lipids, and terpenes. Histological analysis showed that embryogenic P. rupifragum culture is characterized by the formation of complexes of fused embryoids (CFE), which presumably arise either as a result of cleavage polyembryony or during the synchronous development of nearby embryoids. A study of the histology and ultrastructure of CFE revealed that the fusion of embryoids is caused by disturbances in the formation of the epidermis and cuticle. Histochemical studies have established that embryogenic P. rupifragum culture synthesizes and accumulates triacylglycerides, polysaccharides, phenolic compounds (PCs), terpenes, and alkaloids. It has been shown that the quantitative and qualitative composition of the PCs and alkaloids of the P. rupifragum culture depended on the age of the culture and its differentiation, adjustable by growing conditions (light, dark). Differentiated embryogenic P. rupifragum culture retains the ability to form embryoids on a hormone-free MS medium for a long time of cultivation and can be the basis for the further development of biotechnological methods for producing medicinal compounds for cosmetology and pharmacology.







Similar content being viewed by others
REFERENCES
Fazili, M.A., Bashir, I., Ahmad, M., Yaqoob, U., and Geelani, S.N., In vitro strategies for the enhancement of secondary metabolite production in plants: a review, Bulletin of the National Research Centre, 2022, vol. 46, p. 1. https://doi.org/10.1186/s42269-022-00717-z
Butnariu, M.M., Quispe, C., Herrera-Bravo, J., Pentea, M., Sarac, I., Küşümler, A.S., Özçelik, B., Painuli, S., Semwal, P., Imran, M., Gondal, T.A., Emamzadeh-Yazdi, S., Lapava, N., Yousaf, Z., Kumar, M., et al., Papaver plants: current insights on phytochemical and nutritional composition along with biotechnological applications, Oxid. Med. Cell. Long., 2022 vol. 2022, p. 1. https://doi.org/10.1155/2022/2041769
Nessler, C.L., Somatic embryogenesis in the opium poppy, Papaver somniferum, Physiol. Plant., 1982, vol. 55, p. 453. https://doi.org/10.1111/j.1399-3054.1982.tb04526.x
Kassem, M.A. and Jacquin, A., Somatic embryogenesis, rhizogenesis, and morphinan alkaloids production in two species of opium poppy, J. Biomed. Biotech., 2001, vol. 1, p. 70. https://doi.org/10.1155/S1110724301000237
Kutchan, T.M., Ayabe, S., Krueger, R.J., Coscia, E.M., and Coscia, C.J., Cytodifferentiation and alkaloid accumulation in cultured cells of Papaver bracteatum, Plant Cell Reports, 1983, vol. 2, p. 281. https://doi.org/10.1007/BF00270181
Kunakh, V.A., Papaver somniferum L. and Papaver bracteatum Lindl., in: Biotech. Med. Plants. Genetic, physiological and biochemical basis, Kiev: Logos, 2005, p. 516.
Murashige, T. and Skoog, F., A revised medium for rapid growth and bio assays with tobacco tissue cultures, Physiol. Plant., 1962, vol. 15, p. 473. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Betekhtin, A., Rojek, M., Jaskowiak, J., Milewska-Hendel, A., Kwasniewska, J., Kostyukova, Y., Kurczynska, E., Rumyantseva, N., and Hasterok, R., Nuclear genome stability in long-term cultivated callus lines of Fagopyrum tataricum (L.) Gaertn, PLoS ONE, 2017, vol. 12, p. e0173537 https://doi.org/10.1371/journal.pone.0173537
Akulov, A.N. and Kostyukova, Yu.A., Cultivation conditions, histological and biochemical analysis of callus culture of licorice Glycyrrhiza glabra L., Cytology, 2021, vol. 63, p. 590. https://doi.org/10.31857/S004137712106002X
Folin, O. and Ciocalteu, V., On tyrosine and tryptophane determinations in proteins, J. Biol. Chem., 1927, vol. 73, p. 627. https://doi.org/10.1016/S0021-9258(18)84277-6
Wagner, H. and Bladt, S., Plant drug analysis: a thin layer chromatography atlas. 2nd ed. Berlin, New York: Springer, 1996.
Šamaj, J., Bobák, M., Blehová, A., Krištin, J., and Auxtová-Šamajová, O., Developmental SEM observations on an extracellular matrix in embryogenic calli of Drosera rotundifolia and Zea mays, Protoplasma, 1995, vol. 186, p. 45. https://doi.org/10.1007/BF01276934
Facchini, P.J. and Bird, D.A., Developmental regulation of benzylisoquinoline alkaloid biosynthesis in opium poppy plants and tissue cultures, In Vitro Cell. Develop. Biol. Plant., 1998, vol. 34, p. 69. https://doi.org/10.1007/BF02823126
Della Rocca, G., Papini, A., Posarelli, I., Barberini, S., Tani, C., Danti, R., and Moricca, S., Ultrastructure of terpene and polyphenol synthesis in the bark of Cupressus sempervirens after Seiridium cardinale infection, Front. Microbiol., 2022, vol. 13, p. 1. https://doi.org/10.3389/fmicb.2022.886331
Alcantara, J., Bird, D.A., Franceschi, V.R., and Facchini, P.J., Sanguinarine biosynthesis is associated with the endoplasmic reticulum in cultured opium poppy cells after elicitor treatment, Plant Physiol., 2005, vol. 138, p. 173. https://doi.org/10.1104/pp.105.059287
Zhang, N., Wang, M., Li, Y., Zhou, M., Wu, T., and Cheng, Z., TLC–MS identification of alkaloids in Leonuri herba and Leonuri fructus aided by a newly developed universal derivatisation reagent optimised by the response surface method, Phytochem. Analys., 2021, vol. 32, p. 242. https://doi.org/10.1002/pca.2970
Sieber, P., Schorderet, M., Ryser, U., Buchala, A., Kolattukudy, P., Métraux, J.-P., and Nawrath, C., Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions, Plant Cell., 2000, vol. 12, p. 721. https://doi.org/10.1105/tpc.12.5.721
Javelle, M., Vernoud, V., Rogowsky, P.M., and Ingram, G.C., Epidermis: the formation and functions of a fundamental plant tissue, New Phytol., 2011, vol. 189, p. 17. https://doi.org/10.1111/j.1469-8137.2010.03514.x
Batygina, T.B. and Vinogradova, G.Yu., The phenomenon of polyembryony. Genetic heterogeneity of seeds, Ontogenesis, 2007, vol. 38, No. 7, p. 166.
Filonova, L.H., Bozhkov, P.V., and Arnold, S. von., Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking, J. Exp. Bot., 2000, vol. 5, p. 249. https://doi.org/10.1093/jexbot/51.343.249
Rumyantseva, N.I., Arabinogalactan proteins: participation in plant growth and morphogenesis, Biochemistry, 2005, vol. 70, No. 10, p. 1301.
Rumyantseva, N.I., Šamaj, Y., Enzikat, H.-Y., Salnikov, V.V., Baluška F. and Volkmann, D., Changes in the extracellular matrix surface network during cyclic reproduction of proembryonic cell complexes in the Fagopyrum tataricum (L.) Gaertn callus Dokl. Akad. Nauk, 2003, vol. 391, no. 1, p. 123.
Konieczny, R., Bohdanowicz, J., Czaplicki, A., and Przywara, L., Extracellular matrix surface network during plant regeneration in wheat anther culture, Plant Cell Tiss. Organ Cult., 2005, vol. 83. p. 201. https://doi.org/10.1007/s11240-005-5771-9
Hazarika, B., Morpho-physiological disorders in in vitro culture of plants, Scientia Horticulturae, 2006, vol. 108, p. 105. https://doi.org/10.1016/j.scienta.2006.01.038
Kerstiens, G., Effects of low light intensity and high air humidity on morphology and permeability of plant cuticles, with special respect to plants cultured in vitro, In: Physiol. Growth Develop. Plants Cult., Lumsden, P.J., Nicholas, J.R., and Davies, W.J., Eds., Dordrecht: Springer Netherlands, 1994, p. 132.
Johansson, M., Kronestedt-Robards, E., and Robards, A., Rose leaf structure in relation to different stages of micropropagation, Protoplasma, 1992, vol. 166, p. 165. https://doi.org/10.1007/BF01322779
Wetzstein, H. and Sommer, H., Leaf anatomy of tissue-cultured Liquidambar styraciflua (Hamamelidaceae) during acclimatization, Amer. J. Bot., 1982, vol. 69, p. 1579. https://doi.org/10.1002/j.1537-2197.1982.tb13411.x
Matas, A., Lopez-Casado, G., Cuartero, J., and Heredia, A., Relative humidity and temperature modify the mechanical properties of isolated tomato fruit cuticles, Amer. J. Bot., 2005, vol. 92, p. 462. https://doi.org/10.3732/ajb.92.3.462
Schreiber, L., Skrabs, M., Hartmann, K., Diamantopoulos, P., Simanova, E., and Santrucek, J., Effect of humidity on cuticular water permeability of isolated cuticular membranes and leaf disks, Planta, 2001, vol. 214, p. 274. https://doi.org/10.1007/s004250100615
Yeung, E., The orchid embryo—“an embryonic protocorm”, Botany, 2022, vol. 100, p. 691. https://doi.org/10.1139/cjb-2022-0017
Schuchmann, R. and Wellmann, E., Somatic embryogenesis of tissue cultures of Papaver somniferum and Papaver orientale and its relationship to alkaloid and lipid metabolism, Plant Cell Reports, 1983, vol. 2, p. 88. https://doi.org/10.1007/BF00270173
Lančaričová, A., Havrlentová, M., Muchová, D., and Bednárová, A., Oil content and fatty acids composition of poppy seeds cultivated in two localities of Slovakia, Agriculture (Polnohospodárstvo), 2016, vol. 62, p. 19. https://doi.org/10.1515/agri-2016-0003
Christodoulakis, N., Tsiarta, M., and Fasseas, C., Leaf structure and histochemical investigation in Papaver rhoeas L. (Corn Poppy, Field Poppy), J. Herbs, Spices Med. Plants, 2013, vol. 19, p. 119. https://doi.org/10.1080/10496475.2012.755942
Singh, H., Batish, D., Kaur, S., Arora, K., and Kohli, K., α-Pinene inhibits growth and induces oxidative stress in roots, Ann. Bot., 2006, vol. 98, p. 1261. https://doi.org/10.1093/aob/mcl213
Slavíková, L. and Slavík, J., Alkaloids from Papaver rupifragum Boiss. & Reut., Collect. Czech. Chem. Commun., 1980, vol. 45, p. 761. https://doi.org/10.1135/cccc19800761
Galewsky, S. and Nessler, C., Synthesis of morphinane alkaloids during opium poppy somatic embryogenesis, Plant Sci., 1986, vol. 45, p. 215. https://doi.org/10.1016/0168-9452(86)90142-1
Kamo, K. and Mahlberg, P., Morphinan alkaloids: biosynthesis in plant (Papaver spp.) tissue cultures, Medic. Aromat. Plants I, Bajaj, Y.P.S., Ed., Berlin: Springer Heidelberg, 1988, vol. 4, p. 251.
Kunakh, V.A. and Katsan, V.A., Biosynthesis of poppy isoquinoline alkaloids in nature and culture in vitro 1. Poppy soporific Papaver bracteatum L., Ukr. biochem. zh., 2003, vol. 75, No. 5, p. 41.
Hagel, J., Yeung, E., and Facchini, P., Got milk? The secret life of laticifers: 12, Trends Plant Sci., 2008, vol. 13, p. 631. https://doi.org/10.1016/j.tplants.2008.09.005
Krasteva, G., Georgiev, V., and Pavlov, A., Recent applications of plant cell culture technology in cosmetics and foods, Engin. Life Sci., 2021, vol. 21, p. 68. https://doi.org/10.1002/elsc.202000078
Funding
The research was carried out with the financial support of the Ministry of Science and Higher Education of the Russian Federation within the framework of government assignments carried out by Kazan Institute of Biochemistry and Biophysics, Federal Research Center Kazan Scientific Center, Russian Academy of Sciences (state registration number 122011800137-0).
The work was partially performed using equipment at the Collective spectro-analytical center for the study of the structure, composition, and properties of substances and materials of the Federal research center of the Kazan scientific center of the Russian academy of sciences (Hitachi 7800 electron microscope).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations: CFE—complex of fused embryoids; ECMSN—extracellular matrix surface network; MS medium—Murashige and Skoog medium; TBO—toluidine blue; TEM—transmission electron microscopy; PCs—phenolic compounds.
Rights and permissions
About this article
Cite this article
Rumyantseva, N.I., Kostyukova, Y.A., Valieva, A.I. et al. Establishing a Long-Term Cultivated Embryogenic Culture of Papaver rupifragum Boiss. & Reut. and Its Cytological and Biochemical Study. Russ J Plant Physiol 70, 167 (2023). https://doi.org/10.1134/S102144372370022X
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S102144372370022X