Abstract
The genus Taxus (yew) is a source of a number of high-value medicinal substances, particularly, paclitaxel (taxol)—a complex diterpenoid compound with a powerful antitumor action (trade name of Taxol®). Paclitaxel is one of the most efficient drugs in chemotherapy owing to its specific ability to suppress proliferation of tumor cells via stabilization of their microtubules. The world-wide demand for taxol is 800–1000 kg a year and these figures annually rise by 20%. The growing need for paclitaxel and its derivatives and the shortage of plant resources necessary for their production made compounds of the taxane group one of the most important objects for development of biotechnological methods of their production. Out of all the possible ways of taxol production (isolation from wild or plantation trees, total chemical synthesis or semisynthesis, use of yew cell cultures, techniques of metabolic engineering, and use of yew endophytic fungi), the most promising is industrial cultivation of Taxus spp. cell cultures. This review examines the papers dealing with investigation of secondary metabolism in dedifferentiated cells in vitro of various yew species and feasibility of industrial use of cell cultures for production of taxoids. We revealed a number of specificity of Taxus spp. cell cultures: (1) from a cytophysiological aspect—difficult initiation of cell cultures, their low growth characteristics, specific media and culturing conditions; (2) from a phytochemical aspect—distinction from intact plants in qualitative composition and content of secondary metabolites accounted for by specificity of cell culture as a biological system; predominant formation of С14-hydroxylated rather than of С13-hydroxylated taxoids; an opportunity for elevation of the content of taxoids—including commercially valuable ones (paclitaxel and baccatin III) with the aid of different tools (elicitation, stress exposures, two-phase cultivation and some others); (3) from a biotechnological aspect—possibility of industrial cultivation of yew cell cultures; existence of several successful industries (Germany and the Republic of Korea).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The genus Taxus, known as yew, belongs to gymnosperms from the family Taxaceae. Yew is a source of valuable medicinal compounds, specifically, of the antitumor drug Taxol® (paclitaxel) [1].
Paclitaxel is one of the most efficient drugs in chemotherapy whose annual sales volume in 2000 exceeded $1.5 billion. However, the history of its application saw a lot of difficulties, it sometimes lost its leading position due to low solubility, structural complexity, and lack of renewable resources for production of pure substance [2].
The annual world demand for taxol is 800–1000 kg, and these figures grow by 20% every year. The content of paclitaxel in yew bark is 0.01–0.03%, whereas a complete course of antitumor treatment takes 2 g of pure substance. Ever-growing demand for yew plants combined with low accumulation of target compound and slow growth of the tree caused a risk of total loss of numerous species of the genus Taxus [3].
At first, paclitaxel was isolated from the bark of Pacific yew (T. brevifolia Nutt.). However, on account of a low level of the target substance (0.01% of bark dry weight), slow growth, and banned export of plant material (imposed because of a mass loss of trees as a result of bark removal), demand for alternative sources of the antitumor substance considerably rose. Alternative ways of taxol production comprise field growing, total chemical synthesis, semisynthesis (chemical synthesis from baccatin III and 10-deacetylbaccatin III), in vitro cell cultures of Taxus spp., metabolic engineering of producers (including bacterial and fungal) and use of yew endophytic fungi [4, 5].
In 1991, investigation of endophytic microorganisms of T. brevifolia was launched in order to find a fungus or bacterium capable of producing paclitaxel de novo. Although endophytic fungi of yew could produce the desired compound, the rate of production and concentration of the substance remained very low [5, 6].
In 1994, a total chemical synthesis of paclitaxel was reported [5]. Its biosynthesis in a plant is believed to comprise approximately 19–20 steps [7–9]. Paclitaxel is a complex molecule containing more than 11 chiral centers and a unique oxetane ring; its complete organic synthesis has not yet ensured any commercially feasible methods for the compound’s production. In the 1990s, paclitaxel intermediate 10-deacetylbaccatin III was chemically modified into a semisynthetic taxol, this method was used by Bristol Myers Squibb for more than 10 years as the main technique of this drug’s production [5, 10].
Cell cultures of Taxus spp. are a promising tool of production of high-quality plant material for isolation of paclitaxel [8]. In 1991, the first patent was issued on suspension culture of T. brevifolia to produce taxol (with declared yield of 1–3 mg/L). In 1995, Phyton Biotech licensed its process of production of the substance for Bristol Myers Squibb. At present, paclitaxel is supplied on the world market by Phyton Biotech (Germany) [11].
The aim of this review is to examine the main principles of secondary metabolism in cell culture of Taxus spp.
TAXONOMY AND DISTRIBUTION OF PLANTS FROM THE GENUS Taxus
The genus Taxus includes coniferous trees or shrubs from the family Taxaceae 3–9-m-high, rarely up to 25 m. Different species of yew are often found in the temperate zone throughout the world, especially on the Pacific and Atlantic coasts; in the region of the Great Lakes in North America; in West, North, and South Europe; Algeria; in southeast Russia; in East China; Nepal; Burma; Laos; Vietnam; Iran; and on the islands of Sumatra and Celebes [12, 13].
T. baccata L. was the first yew species described by Carl Linnaeus in 1753. To date, 24 species and 55 varieties of the genus Taxus are distinguished by morphological characteristics [12, 13].
The species are subdivided into three groups by differences in epidermal and stomatal leaf traits. The group Wallichiana comprising 11 species is found from the Central Himalayas to Indonesia and Philippines, in North America (northwest coast of the Pacific Ocean) and from Mexico to Central America with isolated strips in Florida; the group Baccata with 9 species occurs in temperate Eurasia, North Africa and the eastern part of North America; the group Sumatrana with 4 species shares the territory with Wallichiana in Asia but is not found in North America [12, 14].
Although 24 species and 55 varieties of the taxon may be distinguished by morphological characteristics, this classification remains disputable. A more conservative taxonomic approach recognizes only 7 to 11 species that are separated geographically: T. baccata, T. brevifolia, T. canadensis Marshall., T. chinensis Rehd., T. cuspidata Siebold. et Zucc. ex Endl., T. floridana Nutt. ex Chapm., T. fauna Nan Li and RR Mill, T. globosa Schltdl., T. mairei S.Y.Hu, T. sumatrana de Laub., and T. wallichiana Zucc. [12–15].
SECONDARY METABOLITES OF Taxus spp.
More than 500 taxoids (taxanes)—diterpenoid compounds of specific structure—were isolated from different species of yew along with “taxine alkaloids”, flavonoids and lignans. The main secondary metabolites of Taxus spp. are taxanes. The most popular compound in this group is paclitaxel, which was isolated for the first time from the bark of T. brevifolia in 1971 [10].
Taxoids (Taxanes)
Classification of taxanes comprises 11 types of compounds and rests on the location of their ring systems. Common taxanes 6/8/6 (figures show the size of every ring) have a linear tricyclic ring system. Other tricyclic ring systems were detected in 11(15→1)-abeotaxanes (5/7/6), 11(15→1), (10→9)-diabeotaxanes (5/6/6) and 2(3→20)-abeotaxanes (6/10/6). Tetracyclic ring systems are characteristic of 3,11-cyclotaxanes (6/5/5/6) and 14,20-cyclotaxanes (6/8/6/6). 3,11:12,20-dicyclotaxanes (6/5/5/5/6) and 3,11:4,12-dicyclotaxanes (6/5/5/4/6) have pentacyclic skeletons. The hexacyclic ring system occurs in 3,11:4,12:14,20-tricyclotaxanes (5/5/4/6/6/6). Bicyclic ring systems are typical of 3,8-secotaxanes (6/12) and 11,12-secotaxanes (8/6) [10, 16].
Metabolites with a 6/8/6 ring system (nomenclature is based on the number of carbon atoms in rings A, B and C) form the most common class of taxanes and have 349 known structures occurring in the genus Taxus. Common 6/8/6 taxanes are subdivided into 12 subgroups depending on the functional group added to the taxane core. Most of the 6/8/6 taxanes (11 subgroups) are oxygenated or hydroxylated at the C-13 position and only one subgroup comprises taxoids oxygenated at С-14 (these compounds have no functional group at position С-13). Paclitaxel belongs to the 6/8/6 taxanes with an oxetane ring and a phenylisoserine lateral chain at the C‑13 position (Fig. 1). Metabolites with a 5/7/6 ring system (11(15→1)-abeotaxanes) form another numerous class of taxanes; approximately 127 structures are known in representatives of the genus Taxus [10, 16].
In all the examined species of yew, 6/8/6-taxanes and 11(15→1)-abeotaxanes were found, while 2(3→20)-abeotaxanes occur in all the species of genus Taxus, except those three where they have not been detected yet: T. brevifolia, T. chinensis, and T. wallichiana. Secotaxanes were described in T. canadensis, T. chinensis, T. cuspidata, T. mairei and T. sumatrana. Cyclotaxanes were found in T. baccata, T. canadensis, T. cuspidata and T. yunnanensis Cheng et L.K.Fu. Diabeotaxanes were isolated from only three yew species: T. sumatrana, T. wallichiana and T. yunnanensis [10, 16].
It is assumed that taxane classes with few members usually occur in not numerous species of the genus Taxus. Moreover, low taxane concentrations in certain classes may account for the fact that they were not found in the majority of yew species [10, 16].
The formation of diterpenoid skeleton of taxoids starts with the production of isopentenyl diphosphate and dimethylallyl diphosphate in plastids via 2-C-methyl-D-erythritol phosphate (MEP) pathway. Cyclization of geranylgeranyl diphosphate by taxadiene synthase results in the formation of taxadiene (the main structure of the taxane ring). Subsequently, the taxane ring undergoes a series of oxidative modifications, acetylation and hydroxylation and is converted into a genuine compound. Biosynthesis of taxol and similar taxoids comprises approximately 19–20 stages and involves 16 enzymes [9].
Phenolic Compounds
Taxus spp. contains a wide range of lignans. Approximately 50 compounds of this class were isolated from yews, including neolignans and a few terpenolignans. In particular, T. wallichiana contained isoliovil, conidendrin, matairesinol, taxiresinol, (–)-secoisolariciresinol, isotaxiresinol, (−)-7′-О-methyl tanegol, formosanol, (+)-tsugacetal, α-intermedianol, oxabicyclooctalignan and lanceolatanin. Five lignans that are dimeric phenylpropanoids were identified in T. baccata: lariciresinol, 3′-demethylisolariciresinol-9′-hydroxy isopropyl ether, taxiresinol and 3-demethylisolariciresinol (Fig. 1) [17, 18].
Different species of yew also contain a variety of flavonoids. Scyadopitysin, amentoflavone, ginkgetin, luteolin, taxifolin, apigenin, isorhamnetin, robustaflavone, etc., were identified in Taxus spp. plants. In different species of yew, branches and leaves are of great interest since they accumulate much isoquercitrin, quercitrin, bilobetin and scyadopitysin [19]. Fourteen flavonoids were identified on the surface of T. baccata needles, where predominant substances were 3-О-rutinoside myricetin, 3-О-rutinoside quercetin and quercetin. Directly from the needles of T. baccata, 3-О-rutinoside quercetin, 7-О-glucoside kaempferol and quercetin, myricetin, quercetin and kaempferol were isolated (Fig. 1) [20, 21].
Taxines (“Taxine Alkaloids”)
All parts of Taxus spp. plants (except for aril) are poisonous both fresh and dry because of the so-called “taxine alkaloids” (taxines). According to the present-day classification, taxines are pseudo-alkaloids since they are formed from nitrogen-free polyhydroxyl diterpenes (taxicines) esterified with β-dimethylamino-β-phenylpropionic and acetic acids. There exist two main groups of taxines: taxine A and taxine B. Structural analog of taxine A (2-deacetyl taxine A) was isolated from the leaves of T. baccata in 1994. Preliminary structure of taxine B was described for the first time in 1986 but its molecular and structural formulas were devised in 1991. Isotaxine B (a structural isomer of taxine B) is the main component of alkaloid fractions of different species of yew (Fig. 1) [22].
BIOLOGICAL ACTIVITY OF SECONDARY METABOLITES OF Taxus spp.
Biological Activity of Taxoids
Paclitaxel (taxol) is an antitumor drug that affects stabilization of microtubules it is a chemotherapeutic agent widely used for treatment of numerous oncological diseases. Mechanisms of taxol action related to suppression of tumor growth may operate on different levels: the drug initiates numerous signal pathways leading to programmed cell death; it may regulate expression of certain microRNAs associated with cancer progression; it produces numerous beneficial effects on modulation of immune response via regulation of chemokines, cytokines or immune cells [5]. In contrast to other tubulin-binding antitumor drugs that prevent tubulin assembly and formation of microtubules, paclitaxel promotes tubulin assembly, production of microtubules and prevents their dissociation, which results in stabilization of microtubules in G2-М stages of the cell cycle, their depolymerization into soluble tubulin is blocked and cell proliferation is suppressed [23].
Paclitaxel was discovered in 1971 and the U.S. Food and Drug Administration (FDA) approved its application for treatment of advanced ovarian cancer in 1992 after a series of clinical trials. Since then, taxol has been widely used in chemotherapy of breast cancer, colorectal cancer and epidermoid cancer of urinary bladder. In addition, it was used for treatment of such diseases as head and neck cancer, small cell and nonsmall cell carcinoma of lung and Kaposi’s sarcoma associated with AIDS [23].
At present, in addition to taxol, there exist a number of its semisynthetic analogs, such as docetaxel and cabazitaxel that are also widely used as chemotherapeutic agents [24].
Biological Activity of Phenolic Compounds
Yew lignans possess an antitumor potential. Taxiresinol isolated from T. wallichiana showed such an activity in respect of ovarian, colorectal, breast and liver cancer in vitro. Three lignans (secoisolariciresinol, taxiresinol and isotaxiresinol) isolated as main components from the wood of T. yunnanensis were tested for antiproliferative activity against cell lines of mouse colorectal cancer and human fibrosarcoma; among examined compounds, the greatest activity was shown by secoisolariciresinol in respect to fibrosarcoma [17].
Isotaxiresinol and secoisolariciresinol from T. yunnanensis wood very actively eliminated DPPH radicals and produced a profound inhibitory effect on the production of nitrogen oxide. Both lignans prevented liver injury caused by D-galactosamine/lipopolysaccharide via suppression of apoptosis of hepatocytes by means of blocking TNF-α and producing IFN-γ [17].
Isotaxiresinol, secoisolariciresinol and taxiresinol were tested for hypoglycemic action. At a dose of 100 mg/kg (administered intravenously), isotaxiresinol lowered fasting blood glucose level in diabetic rats by 34.5%, whereas secoisolariciresinol and taxiresinol reduced it by 33.4 and 20.9%, respectively. Lignans from T. baccata moderately inhibited butyrylcholin esterase and lipoxygenase involved in pathogenesis of Alzheimer’s disease [17].
High antiradical activity was shown for flavonoids from T. wallichiana, T. cuspidata and T. baccata. Needles of T. baccata produced hepatoprotective, tranquilizing and sedative effects presumably accounted for by benzodiazepine-like action of flavonoids detected therein. Scyadopitysin showed neuroprotection against injuries of primary cortical neurons caused by Aβ-protein [20, 21, 25–27].
Biological Activity of “Taxine Alkaloids”
Disturbance of cardiac activity caused by yew intoxication is mainly attributed to the action of the so-called “taxine alkaloids” modifying permeability of myocardium cells to sodium and calcium via blocking sodium and calcium channels. Taxines impair sodium-potassium transport and bring about a rise in cytoplasmic calcium in the cells, which stimulates the onset and progression of ominous arrhythmia. Dizziness, mydriasis, nausea, vomiting, pain in the abdominal region, tachycardia and convulsions are followed by bradycardia, paralysis, diastolic cardiac arrest and death [28]. “Taxine alkaloids” are rapidly absorbed in the digestive system, and the symptoms of poisoning show in 30–90 min [29]. Taxine B administered in vivo or in vitro was more cardiotoxic than taxine A. The mechanism of action of taxine B is identical to class I antiarrhythmic drugs, such as flecainide, procainamide and quinidine [22].
CELL CULTURES OF Taxus spp. AND FORMATION OF SECONDARY METABOLITES THEREIN
Initiation of Taxus spp. Cell Cultures
Among higher plants, conifers are the most difficult objects for initiation of cell culture. In Taxus spp., the task is complicated by a high probability of the presence in the explants of a large quantity of secondary metabolites that may be rather toxic [30].
Callus cell culture of yew (Т. baccata) was produced for the first time in 1973. Subsequently, numerous researchers made efforts to optimize conditions of callusogenesis and cultivation of the obtained cell cultures of different yew species with special attention being paid to the choice of explant, the methods of its sterilization, optimization of medium composition and culturing conditions [1, 4, 31].
A number of researchers reported that frequency and efficiency of callus formation depended on the phenological state of the donor plant, however, the results were ambiguous. In one case, when the needles of T. baccata and T. canadensis were used as explants, the greatest frequency of callusogenesis was observed in plant material collected in July–August, whereas the frequency of callus formation was three times lower and the time interval before the onset of callus formation was two times longer in the needles gathered in spring (March–April) and autumn (September–November) [31]. In another investigation, the best results were obtained with “winter” explants (January) as compared with summer material (July). The authors relate the dependence of efficiency of callusogenesis on the phenological state of the donor plant to different levels of phenolic compounds in explants: their level is much lower in “winter” needles [32].
Many researchers note that “darkening” of calli caused by the production of large quantity of phenolic compounds in the course of the initiation of yew cell cultures occurs rather often [33]. This may lead to a death of cells, and special arrangements should be made to avoid this process.
In order to inactivate free phenols and improve growth of calli, some authors supplemented nutrient medium with antioxidants—ascorbic acid (10–100 mg/L) or polyvinyl pyrrolidone (0.1–0.5%), and activated charcoal (0.1–1.0%) as a sorbent. Experiments with several species of yew have shown that addition of polyvinyl pyrrolidone removed darkening of nutrient medium but only slightly reduced necrotization of callus; the introduction in medium of activated charcoal decreased callus necrotization three to four times as compared with control material but sevenfold reduced the frequency of callusogenesis; the addition of ascorbic acid decreased necrotization of explant and primary callus twofold and increased the rate of callus growth [31].
The most important components of nutrient medium affecting callusogenesis are phytohormones. Numerous researchers investigated the effect of hormonal composition of the media on frequency of callus formation. In particular, T. baccata needles used as explants showed the greatest efficiency of callusogenesis on Gamborg medium supplemented with 2 mg/L 2,4-D and on Westcott medium for conifers supplemented with α-naphthylacetic acid (α-NAA, 4 mg/L) and BAP (0.5 mg/L) [31]. However, available data suggest that the optimal hormonal composition of the medium for callusogenesis in Taxus spp. has not been devised; in each case, an individual composition should be selected.
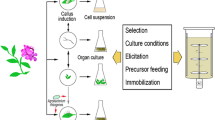
Suspension cell cultures of Taxus spp. are mainly produced by means of the transfer of primary callus to a liquid nutrient medium and they predominantly consist of small cell aggregations. Some papers report about a high variability in growth parameters and physiological characteristics of suspension cell cultures produced from callus cultures and from explants of the same kind from different plant species [1, 4, 8].
Optimization of Cultivation of Taxus spp. Cell Cultures
The efficiency of cultivation in vitro depends a lot on the correct choice of nutrient medium [30]. In the beginning, the majority of investigations with both callus and suspension cell cultures of different species of the genus Taxus failed to obtain highly productive lines that could be immediately used for industrial production of paclitaxel. Early investigations showed that the cells of Taxus spp. in vitro can synthesize taxol and similar taxoids under optimized conditions. In callus culture of T. baccata grown on MS medium supplemented with different growth regulators, eight analogs of paclitaxel were identified [1, 2, 8].
Elevated productivity of cell cultures is usually achieved by the selection of lines with a high content of taxol. The main disadvantage associated with this approach is slow accumulation of biomass. According to some authors, the doubling of biomass in T. brevifolia and T. cuspidata cell cultures occurs by the 14th–20th day, which is five to six times slower that in the majority of other cell cultures [8, 34].
Growth of cell culture and biosynthesis of target compounds therein considerably depend on carbohydrate composition of the nutrient medium. Some researchers looked into the effect of carbohydrate concentration on the production of taxoids in the cells of Taxus spp. in vitro. These investigations showed that the content of taxol was 0.02% on a dry weight basis, which is comparable to the concentration of this compound in the bark of intact plants. It is interesting that individual carbohydrates differently affect paclitaxel synthesis in cell culture of yew, which intensified in the presence of fructose and was suppressed by glucose [1, 8].
Numerous investigations conducted in different laboratories resulted in the initiation of callus and suspension cell cultures of different species of Taxus with satisfactory growth characteristics [35]. Cell cultures efficiently producing taxoids were initiated for yew plants of different species: T. baccata, T. media (hybrid between T. baccata and T. cuspidata), T. cuspidata, T. brevifolia, T. chinensis, T. globosa and T. wallichiana [1, 4, 8, 36, 37].
It is interesting that cell cultures of Taxus spp. hold a sort of record deserving of Guinness World Records: several actively growing cultures were initiated from a T. baccata tree growing in Nikitsky Botanical Gardens (Republic of Crimea) whose age is at least 800 years (some experts estimate it as approximately 1000 years). Available sources of information suggest that it is currently the oldest plant that gave rise to cell cultures (Figs. 2, 3) [38].
Formation of Taxoids in Cell Cultures of Taxus spp.
The majority of papers deal with the investigation of cell cultures of different Taxus species from the aspect of formation of taxoids therein. The works mostly use callus cell cultures where paclitaxel is searched for and identified. There are literature reports about the initiation of cell cultures containing taxol in the amount comparable to its content in intact plants, but most authors report about its absence or traces [2, 4, 35, 36]. In suspension culture, paclitaxel was for the first time found in 1989 (cell culture of T. brevifolia) [39].
Most works examine the production in cell cultures of commercially important С13-hydroxylated taxoids, paclitaxel and baccatin III, whereas the formation of С14-hydroxylated taxoids in cell cultures is poorly investigated. However, available results suggest that cell cultures of yew usually produce exactly this group of compounds and their qualitative composition is more diverse and the content much greater than of 13-ОН derivatives [40]. Intact plants of Taxus spp. are believed to predominantly accumulate 13-hydroxylated taxoids, however, the content of different groups of taxanes in different plant organs considerably differs [10, 16].
It should be noted that toxicity of 14-hydroxylated compounds is much lower than of 13-ОН derivatives, and this fact may account for their preferred production in proliferating dedifferentiated plant cells in vitro [41].
Intensification of Production of Pharmaceutically Valuable Taxoids in Taxus spp. Cell Cultures
Effect of signal substances (phytohormones, growth regulators and elicitors). One of the well-examined stimulators of paclitaxel formation in Taxus spp. cell cultures is phytohormone methyl jasmonate (MeJA) that some authors incorrectly attribute to elicitors [4]. MeJA was used for the first time in cell cultures of T. baccata in 1996 when it was found to intensify synthesis of taxoids more than 120 times. Methyl jasmonate was used for intensification of paclitaxel production in cell cultures of T. baccata, T. canadensis and T. cuspidata [36, 42–44].
After a stimulation with 100 µM MeJA, callus culture of T. cuspidata produced five taxoids (cephalomannine, 1β-dehydroxybaccatin VI, taxinine NN-11, baccatin I and 2α-acetoxytaxusin) and one abietane—taxamairin C—in addition to already present compounds, such as paclitaxel, 7-epi-taxol, taxol С, baccatin VI, taxayuntin С, taxuyunnanin С and its analogs, yunnanxane and taxamairin А. These results indicate that MeJA may modify the qualitative composition of taxoids affecting certain biosynthetic pathways in plant cells in vitro [45].
The effect of MeJA may become more pronounced in combination with other stimulators of biosynthesis of secondary metabolites. The combination of chitosan, MeJA and Ag+ brought about a rise in the production of paclitaxel in suspension culture of T. chinensis, which was almost 40 times greater than in control culture (without elicitors), ten times higher than in the culture exposed to Ag+ alone, six times higher than in the culture with only chitosan added and two times greater than in the presence of MeJA alone [46]. Elicitor from the fungus Rhyzopus stelonifera used in combination with methyl jasmonate and salicylic acid intensified production of paclitaxel 16 times when it was added on the 25th–30th day of growth of T. baccata suspension cell culture [47].
The final concentration of MeJA in the medium plays a key role in stimulation of biosynthesis. It was shown that, at a concentration of 200 µM, MeJA stimulates the synthesis of paclitaxel and baccatin III in cell culture of T. baccata less efficiently than at 100 µM [48].
The data are available about application of coronatine, a toxin produced by the pathogen Pseudomonas syringae. Upon exposure to coronatine, total peak concentration of taxoids in suspension culture of T. media was 77.46 mg/L and rose even more greatly than upon the addition of methyl jasmonate (21.48 mg/L), with a control level of 8.14 mg/L [49]. It is interesting that the configuration of the coronatine molecule resembles the structure of jasmonate/isoleucine conjugate and coronatine toxicity largely depends on its jasmonate-like action on plants.
In callus culture of T. cuspidata, paclitaxel concentration rose from 89 to 139 µg/g dry weight after the addition of chitosan, a component of cell walls of some fungi [50]. Production of taxol in suspension cell culture of T. baccata increased approximately three times when nutrient media were supplemented with a complex of amino acids in combination with chitosan [51].
In order to promote synthesis of taxoids, some researchers use vanadyl sulfate, silver nitrate, cobalt chloride, arachidonic acid, ammonium citrate, salicylic acid and polysaccharides of bacterial and fungal origin separately or in combination [36].
It was shown that the content of taxanes in suspension culture of T. media changed upon exposure to perfluorodecalin (a fluorocarbon where all hydrogen atoms are replaced by fluorine) and hexenol (a volatile natural organic compound produced in injured green tissues, which participates in defense interactions between plants) separately and in combination with coronatine and β-cyclodextrins. The total content of taxoids in cell cultures of T. media treated with perfluorodecalin jointly with coronatine and β-cyclodextrins increased 3.3 times. Hexenol added to suspension of T. media blocked the formation of taxol but activated the production of baccatin III [52].
The effect of signal substances (methyl jasmonate, ethanol, buthionine sulfoximine and hydrogen peroxide) on the production of taxoids was estimated in suspension cell culture of T. globosa. The combination of buthionine sulfoximine with hydrogen peroxide brought about a considerable rise in the concentration of 10-deacetylbaccatin (1662 µg/g dry wt), cephalomannine (334.32 µg/g dry wt) and taxol (157 µg /g dry wt) [53].
The efficiency of stimulation of synthesis of pharmaceutically valuable taxoids exposed to signal substances in different suspension cell cultures of Taxus spp. is shown in Table 1.
Biosynthetic precursors. Numerous papers dealt with the effect of precursors, sugars and growth inhibitors on accumulation of paclitaxel in yew cell cultures. Synthesis of deacetylbaccatin III, baccatin III or paclitaxel in cell culture of T. wallichiana intensified upon the addition of phenylalanine, sodium benzoate, hippuric acid and leucine. Growth inhibitors, such as 2‑chloroethyl phosphonic acid and chlorocholine chloride, were good for the production of paclitaxel and deacetylbaccatin III in cell culture of T. wallichiana. Such effects may be associated with the influence of biosynthetic precursors and growth inhibitors on 2α-benzoyl transferase, 10-О-acetyl transferase, phenylpropanoyl transferase and 3-N-debenzoyl-2-deoxytaxol-N-benzoyltransferase and with different responses of these enzymes to external stimuli [54]. A rise in the content of paclitaxel in cell culture of T. cuspidata was observed after introduction of phenylalanine, benzoic acid and N-benzoyl glycine, which are precursors to the side chain of the target compound. Intense production of taxol in the cell culture of T. chinensis was achieved by means of a recurring addition of 3, 1 and 2% sucrose at the beginning of the growth cycle and on the 7th and 21st days, respectively [1].
Physical factors of cultivation. The effect of some physical factors of cultivation (composition of gas mixture, osmotic stress and temperature) on the production of taxoids was investigated in cell cultures of Taxus spp. [1]. It was found that the most optimal composition of gas mixture for the production of paclitaxel in suspension cell culture of T. cuspidata is 10% oxygen : 0.5% carbon dioxide : 5 ppm ethylene [55, 56]. Effect of osmotic stress on the production of taxol was investigated in suspension culture of T. chinensis. The accumulation of paclitaxel was optimal at a starting concentration of sucrose of 60 g/L. At the same time, even osmotic pressure created with mannitol, sorbitol, and polyethylene glycol promoted production of the target compound [1, 57]. Biosynthesis of taxoids in cell culture of yew depended on changes in temperature occurring in the course of culturing. When the temperature rose from 24 to 29°C, on the 21th day of cultivation of T. chinensis suspension cell culture, the yield of paclitaxel rose to 137.5 mg/L (starting content of the target product in the culture was 49.6 mg/L). On the contrary, when temperature changed from 24 to 29°C, the concentration of taxuyunnanin С decreased from 885.9 to 512.9 mg/L [58].
Cell immobilization, various cultivation systems and biotransformation. In some cases, immobilization is a useful tool that boosts the production of secondary metabolites in plant cells in vitro. Calcium alginate used for immobilization of T. baccata cells ensured a threefold rise in production of paclitaxel [1, 4].
Intense production of taxol and many other secondary metabolites in cell cultures is usually observed in the deceleration growth phase and in the stationary phase. A two-stage system of culturing when plant cells are cultivated from the beginning for accumulation of biomass and then transferred to a nutrient medium suitable for production of taxanes is an efficient technique ensuring an increase in production of target compounds. An extra advantage of such a technique consists in the opportunity to add biosynthetic precursors and elicitors when the production of secondary metabolites is at the peak level. This strategy was successfully adopted for boosting paclitaxel and baccatin III production in suspension cell cultures of T. baccata [1, 4].
A small quantity of secondary metabolites excreted to the nutrient medium may disturb biosynthesis of target substances. Creation of the place for accumulation of phytochemical compounds in the form of the second phase (organic solvent or solid compound) makes it possible to produce higher concentrations of pharmaceutically valuable substances. Removal of the products of secondary metabolism from aqueous phase in situ using the two-phase system of culturing facilitates their release from intracellular organelles. It was found that the accumulation of taxol in the cells of in vitro Taxus spp. causes disturbances in the regulation of target compound synthesis and leads to degradation of the product, therefore, its removal from suspension cultures is important for elevation of productivity. Release of taxol and baccatin III from the cells to the medium grew by 120 and 97%, respectively, in the presence of vanadium sulfate. The two-phase cultivation system was successfully employed in cell cultures of T. brevifolia and T. cuspidata [1, 4].
It is interesting to examine biotransformation of compounds of the taxane group since these processes may proceed with the participation of bacteria, fungi, plant cells in vitro and even by isolated enzymes, which may be promising for the production of specific target substances [59].
Industrial Cultivation of Taxus spp. Cell Cultures
To date, commercial production of taxol in industrial bioreactors has been arranged in a number of countries: by Phyton Biotech (Germany), ESCA genetics (California, United States), Phyton (New York, United States) and Samyang Biopharm (Republic of Korea) [4, 11, 36].
Phyton Catalytic (now Phyton Biotech owned by DFB Pharmaceuticals) started scaling-up suspension cell cultures of Taxus chinensis in the beginning of the 1990s in order to launch large-scale production of paclitaxel (Taxol®). Python Biotech (Germany) is currently the largest producer of paclitaxel using cell culture of T. chinensis in large-scale bioreactors with a capacity of up to 75 000 L [2]. The technique of plant cells’ fermentation was licensed for Bristol Myers Squibb in 1995. In 2004, Bristol Myers Squibb received a prestigious Presidential Green Chemistry Challenge Award from the American Chemical Society for switching to cultivation of plants' cell culture as a single source of its anticancer drug Taxol® in 2002 [11].
In 2000, the patent on Taxol® expired, which stimulated competition among manufacturers, including the investigation of new cell cultures of yew as well as development and improvement of the methods of production of paclitaxel and allied taxanes. To date, commercial cultivation of Taxus spp. cell cultures has been realized in the United States (ESCA genetics) and South Korea (Samyang Biopharm). Samyang Biopharm employs cell cultures of Taxus cultivated in 35 000-L bioreactors for the production of paclitaxel under the trade name Genexol® [11].
However, exact information about commercial production of taxoids, including the use of cell cultures, is not available in free access.
CONCLUSION AND PROSPECTS
Natural diterpenoid paclitaxel is one of the most efficient antitumor drugs (Taxol®) with a specific mechanism of action. The traditional source of taxol is bark of Taxus spp. plants, but its low content (~0.02% of dry weight) and difficult isolation and purification make the cost of the final product very high. It is important that the use of wild plants for the production of taxol threatens their existence. The growing demand for paclitaxel and its derivatives under deficiency of plant resources necessary for their isolation account for the fact that compounds of the taxane group have become one of the most important subjects for development of alternative methods of their production, including biotechnological processes [1, 2].
At present, three strategies of this task solution are considered: total chemical synthesis or semisynthesis of target compounds, the search for alternative sources of raw material and application of the methods of genetic and metabolic engineering (creation of transgenic organisms with inserted genes responsible for biosynthesis of taxoids).
Total synthesis and semisynthesis of paclitaxel are usually commercially unprofitable owing to the baffling chemical complexity of the target compound.
The creation of transgenic organisms (bacteria and yeast) may provide a rather efficient way of producing taxoids, however, when the details of their biosynthesis are still unknown it is too early to discuss its prospects. It is tempting to produce cell cultures transgenic by key genes of biosynthesis or cultures of transformed roots (hairy roots), but these systems should probably be attributed to alternative sources of plant material.
Alternative sources of plant material comprise plantation trees, use of endophytic fungi of yew and in vitro cell and organ (transformed or adventitious roots) cultures of Taxus spp. The main disadvantage of plantation growing of yew trees is its duration (several decades). In spite of this significant drawback, this approach is actively realized in China.
Thus, to date the most promising and convenient approach to sustainable production of taxol and similar compounds is industrial cultivation of Taxus spp. cell cultures [5, 8].
In vitro cell culture has numerous advantages: cultivation does not depend on the weather, seasons or environmental pollution; this method ensures continuous production of paclitaxel of sufficient purity from renewable high-quality plant material; it is possible to control the accumulation of target substances in the cultures and increase their productivity by employing different techniques (optimization of composition of nutrient media, use of biosynthetic precursors and elicitors, two-stage and two-phase culturing, metabolic and genetic engineering). However, there are some difficulties: slow cell growth, low yield of target metabolites, high production costs of biotechnological industry [35].
Organization of biotechnological manufacture based on cell cultures of yew primarily depends on the creation of a highly efficient strain-producer the target substances: paclitaxel or other taxoids. In order to put this task into practice, it is important to take into account properties of cell culture as a unique biological system: a population of somatic cells with specific secondary metabolism. This probably accounts for the preferred formation of C14-hydroxylated taxoids in Taxus spp. cell cultures.
To date, a number of biotechnological companies deal with cell cultures of yew. In 1995, Phyton Biotech licensed the process of production of taxol from suspension cell culture of T. chinensis for Bristol Myers Squibb. Such technologies of production of paclitaxel and allied taxanes are now commercially used by Phyton Biotech (Germany), ESCA genetics (California, United States), Phyton (New York, United States) and Samyang Biopharm (Republic of Korea) [4, 11, 36].
Summing up, we reiterate that taxanes are one of the most interesting and promising groups of diterpenoids in the view of chemotherapy of different types of cancer. Investigation of the mechanisms responsible for their biological activity, regulation of biosynthesis (specifically, on molecular-genetic level), search for new alternative sources of the compounds and improvement of the existent technologies are of fundamental and practical importance. Although knowledge in this area is constantly accumulated and deepened, a lot of questions are still open. Progress in molecular genetics associated with investigation of the genes of taxane metabolism and principles of their regulation in intact plant and in cell culture in vitro, as well as a more detailed phytochemical analysis of plant resources and their biotechnological analogs, will probably make it possible to optimize different methods of production of pharmaceutically valuable taxoids.
REFERENCES
Murthy, H.N., Dandin, V.S., Joseph, K.S., Park, S.Y., and Paek, K.Y., Plant cell and organ culture as an alternative for the production of anticancer compounds, in Anticancer Plants: Natural Products and Biotechnological Implements, Akhtar, M.S. and Swamy, M.K., Eds., Singapore: Springer Nature, 2018, p. 429. https://doi.org/10.1007/978-981-10-8064-7_18
Exposito, O., Bonfill, M., Moyano, E., Onrubia, M., Mirjalili, M.H., Cusido, R.M., and Palazon, J., Biotechnological production of taxol and related taxoids: current state and prospects, Anticancer Agents Med. Chem., 2009, vol. 9, p. 109. https://doi.org/10.2174/187152009787047761
Nimasow, G., Nimasow, O.D., Rawat, J.S., and Norbu, L., Conservation efforts of an important medicinal plant (Taxus baccata Linn.) in West Kameng district of Arunachal Pradesh (India), Earth Sci., 2015, vol. 4, p. 1. https://doi.org/10.11648/j.earth.s.2015040301.11
Malik, S., Cusido, R.M., Mirjalili, M.H., Moyano, E., Palazon, J., and Bonfill, M., Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review, Process Biochem., 2011, vol. 46, p. 23. https://doi.org/10.1016/j.procbio.2010.09.004
Zhu, L. and Chen, L., Progress in research on paclitaxel and tumor immunotherapy, Cell. Mol. Biol. Lett., 2019, vol. 24, p. 40. https://doi.org/10.1186/s11658-019-0164-y
Ismaiel, A.A., Ahmed A.S., Hassan I.A., El-Sayed E.S.R., Karam El-Din A.Z.A. Production of paclitaxel with anticancer activity by two local fungal endophytes, Aspergillus fumigatus and Alternaria tenuissima, Appl. Microbiol. Biotechnol., 2017, vol. 101, p. 5831. https://doi.org/10.1007/s00253-017-8354-x
Maheshwari, P., Garg, S., and Kumar, A., Taxoids: biosynthesis and in vitro production, Biotech. Mol. Biol. Rev., 2008, vol. 3, p. 071.
McElroy, C. and Jennewein, S., Taxol® biosynthesis and production: from forests to fermenters, in: Biotechnology of Natural Products, Schwab, W., Lange, B.M., and Wust, M., Eds., Cham: Springer International Publishing, 2018, p. 145. https://doi.org/10.1007/978-3-319-67903-7_7
Wang, T., Li L., Zhuang, W., Zhang, F., Shu, X., Wang, N., and Wang, Z., Recent research progress in taxol biosynthetic pathway and acylation reactions mediated by Taxus acyltransferases, Molecules, 2021, vol. 26, p. 2855. https://doi.org/10.3390/molecules26102855
Wang, Y.F., Shi, Q.W., Dong, M., Kiyota, H., Gu, Y.C., and Cong, B., Natural taxanes: developments since 1828, Chem. Rev., 2011, vol. 111, p. 7652. https://doi.org/10.1021/cr100147u
Wilson, S.A. and Roberts, S.C., Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules, Plant Biotechnol. J., 2012, vol. 10, p. 249. https://doi.org/10.1111/j.1467-7652.2011.00664.x
Spjut, R.W., Taxonomy and nomenclature of Taxus (Taxaceae), J. Bot. Res. Inst. Tex., 2007, vol. 1, p. 203.
Hao, D.C., Xiao, P.G., Ge, G.B., and Liu, M., Biological, chemical, and omics research of Taxus medicinal resources, Drug Dev. Res., 2012, vol. 73, p. 477. https://doi.org/10.1002/ddr.21040
Spjut, R.W., A phytogeographical analysis of Taxus (Taxaceae) based on leaf anatomical characters, J. Bot. Res. Inst. Texas, 2007, vol. 1, p. 291.
Li, J., Davis, C.C., Tredici, P.D., and Donoghue, M.J., Phylogeny and biogeography of Taxus (Taxaceae) inferred from sequences of the internal transcribed spacer region of nuclear ribosomal DNA, Harv. Pap. Bot., 2001, vol. 6, p. 267.
Lange, B.M. and Conner, C.F., Taxanes and taxoids of the genus Taxus—A comprehensive inventory of chemical diversity, Phytochemistry, 2021, vol. 190, p. 112829. https://doi.org/10.1016/j.phytochem.2021.112829
Topcu, G. and Demirkiran, O., Lignans from Taxus species, Top Heterocycl Chem., 2007, vol. 11, p. 103. https://doi.org/10.1007/7081_2007_082
Dang, P.H., Nguyen, H.X., Nguyen, H.H.T., Vo, T.D., Le, T.H., Phan, T.H.N., Nguyen, M.T.T., and Nguyen, N.T., Lignans from the roots of Taxus wallichiana and their α‒glucosidase inhibitory activities, J. Nat. Prod., 2017, vol. 80, p. 1876. https://doi.org/10.1021/acs.jnatprod.7b00171
Krauze-Baranowska, M., Flavonoids from the genus Taxus, Z Naturforsch C J Biosci., 2004, vol. 59, p. 43. https://doi.org/10.1515/znc-2004-1-210
Bekhouche M., Benyammi R., Slaoui M.K., Krimat S., Paris C., Khelifi L., Morsli A. Flavonoid profile and antioxidant properties of Algerian common yew (Taxus baccata L.), Clinical Phytosci., 2022, vol. 8, p. 17. https://doi.org/10.1186/s40816-022-00348-x
Bekhouche, M., Morsli, A., Slaoui, M.K., Benyammi, R., Krimat, S., Paris, C., and Khelifi, L., Antioxidant properties of the needles of Taxus baccata L. growing in Algeria and characterization of their antioxidant constituents by LC–DAD–ESI–MSn, J. Global Trends Pharm. Sci., 2021, vol. 12, p. 9026.
Wilson, C.R. and Hooser, S.B., Toxicity of yew (Taxus spp.) alkaloids, in: Veterinary Toxicology. Basic and Clinical Principles, Gupta, G.S., Ed., USA: Academic Press, 2018, p. 947. https://doi.org/10.1016/B978-0-12-811410-0.00066-0
Sinha, D., A review on taxanes: an important group of anticancer compound obtained from Taxus sp., IJPSR, 2020, vol. 11, p. 1969. https://doi.org/10.13040/IJPSR.0975-8232.11(5).1969-85
Nicolaou K.C. and Valiulin, R.A., Synthesis and biological evaluation of new paclitaxel analogs and discovery of potent antitumor agents, Org. Biomol. Chem., 2013, vol. 11, p. 4154. https://doi.org/10.1039/c3ob40654g
Tabaszewska, M., Antoniewska, A., Rutkowska, J., Skoczylas, L., Slupski, J., and Skoczen-Slupska, R., Bioactive components, volatile profile and in vitro antioxidative properties of Taxus baccata L. red arils, Molecules, 2021, vol. 26, p. 4474. https://doi.org/10.3390/molecules26154474
Bekhouche, M., Benyammi, R., Khelifi Slaoui, M., Khelifi, L., and Morsli, A., Free radical scavenging activity and detailed flavonoid profiling of Algerian yew (Taxus baccata L.) by LC–ESI–MS/MS, IJPSR, 2021, vol. 12, p. 2613. https://doi.org/10.13040/IJPSR.0975-8232.12(5).2613-19
Filipava, S.N., Lohvina, H.O., and Spiridovich, E.V., Organ- and tissue-specific variation in antiradical potential and phenolic compounds content in plants of the genus Taxus spp., Experimental Biology and Biotechnology, 2022, vol. 1, p. 48. https://doi.org/10.33581/2957-5060-2022-1-48-58
Pinto, A., Lemos, T., Silveira, I., and Aragao, I., Taxus baccata intoxication: the sun after the electrical storm, Rev. Bras. Ter. Intensiva., 2021, vol. 33, p. 172. https://doi.org/10.5935/0103-507X.20210019
Valis, M., Koci, J., Tucek, D., Lutonsky, T., Kopova, J., Barton, P., Vysata, O., Krajickova, D., Korabecny, J., Masopust, J., and Klzo, L., Common yew intoxication: a case report, J. Med. Case Rep., 2014, vol. 8, p. 4. https://doi.org/10.1186/1752-1947-8-4
Butenko, R.G., Biologiya kletok vysshikh rasteniy in vitro i biotekhnologii na ikh osnove (Biology of Higher Plant Cells in Vitro and Biotechnologies Based on Them), Moscow: FBK-PRESS, 1999.
Nekora, S.V. and Komov, V.P., Ability to callusogenesis and needle explants of five species of the genus Taxus L., Rastitel’nye Resursy, 2001, vol. 37, p. 100.
Dubravina, G.A., Zaytseva, S.M., and Zagoskina, N.V., Changes in the formation and localization of phenol compounds during dedifferentiation of tissues of European yew and Canadian yew in vitro, Russ. J. Plant Phys., 2005, vol. 52, p. 755.
Zagoskina, N.V., Zaytseva, S.M., and Aleksandrova, M.S., Changes in the formation of phenol compounds upon the introduction of berry yew (Taxus baccata L.) and Canadian yew (Taxus canadensis Marsh.) cells into culture in vitro, Biotekh., 2007, vol. 1, p. 29.
Fett-Neto, A.G., Melanson, S.J., Nicholson, S.A., Pennington, J.J., and DiCosmo, F., Improved taxol yield by aromatic carboxylic acid and amino acid feeding to cell cultures of Taxus cuspidata, Biotech. Bioeng., 1994, vol. 44, p. 967. https://doi.org/10.1002/bit.260440813
Nosov, A.M., Popova, E.V., and Kochkin, D.V., Isoprenoid production via plant cell cultures: biosynthesis, accumulation and scaling-up to bioreactors, in Production of Biomass and Bioactive Compounds Using Bioreactor Technology, Paek, K.Y., Murthy, H.N., and Zhong, J.J., Eds., Dordrecht: Springer, 2014, p. 563. https://doi.org/10.1007/978-94-017-9223-3_23
Cusido, R.M., Onrubia, M., Sabater-Jara, A.B., M-oyano, E., Bonfill, M., Goossens, A., Angeles Pedreno, M., and Palazon, J., A rational approach to improving the biotechnological production of taxanes in plant cell cultures of Taxus spp., Biotechnol. Adv., 2014, vol. 32, p. 1157. https://doi.org/10.1016/j.biotechadv.2014.03.002
Globa, E.B., Demidova, E.V., Gaisinsky, V.V., and Kochkin, D.V., Obtaining and characteristics of callus and suspension plant cell culture of Taxus wallichiana Zucc., Vestnik SVFU, 2018, vol. 2, p. 18. https://doi.org/10.25587/SVFU.2018.64.12127
Globa, E.B., Chernyak, N.D., and Nosov, A.M., Obtaining a cell culture from a unique yew plant Taxus baccata, Materialy Mezhdunarodnoy konferentsii s elementami nauchnoy shkoly dlya molodezhi “Perspektivy fitobiotekhnologii dlya uluchsheniya kachestva zhizni na Severe” (Materials of the International Conference with Elements of a Scientific School for Youth “Perspectives of Phytobiotechnology to Improve the Quality of Life in the North”), Yakutsk, 2010, p. 97.
Ketchum, R.E.B. and Gibson, D.M., Paclitaxel production in suspension cell cultures of Taxus, Plant Cell Tiss. Organ Cult., 1996, vol. 46, p. 9. https://doi.org/10.1007/BF00039691
Kochkin, D.V., Globa, E.B., Demidova, E.V., Gaisinsky, V.V., Galishev, B.A., Kolotyrkina, N.G., Kuznetsov, Vl.V., and Nosov, A.M., Occurrence of 14-hydroxylated taxoids in the plant in vitro cell cultures of different yew species (Taxus spp.), Dokl. Biochem. Biophys., 2017, vol. 476, p. 337. https://doi.org/10.1134/S1607672917050131
Kochkin, D.V., Globa, E.B., Demidova, E.V., Gaisinsky, V.V., Kuznetsov, Vl.V., and Nosov, A.M., Detection of taxuyunnanin C in suspension cell culture of Taxus canadensis, Dokl. Akad. Nauk, 2019, vol. 485, p. 374. https://doi.org/10.31857/S0869-56524853374-376
Bonfill, M., Exposito, O., Moyano, E., Cusido, R.M., Palazon, J., and Pinol, M.T., Manipulation by culture mixing and elicitation of paclitaxel and baccatin III production in Taxus baccata suspension cultures, In Vitro Cell. Dev. Biol. Plant., 2006, vol. 42, p. 422. https://doi.org/10.1079/IVP2006761
Senger, R.S., Phisalaphong, M., Karim, M.N., and Linden, J.C., Development of a culture sub-population induction model: signaling pathways synergy and taxanes production by Taxus Canadensis, Biotechnol. Prog., 2006, vol. 22, p. 1671. https://doi.org/10.1021/bp0602552
Mirjalili, N. and Linden, J.C., Methyl jasmonate induced production of taxol in suspension cultures of Taxus cuspidata: ethylene interaction and induction models, Biotechnol. Prog., 1996, vol. 12, p. 110. https://doi.org/10.1021/bp9500831
Bai, J., Kitabatake, M., Toyoizumi, K., Fu, L., Zhang, S., Dai, J., Sakai, J., Hirose, K., Yamori, T., Tomida, A., Tsuruo, T., and Ando, M., Production of biologically active taxoids by a callus culture of Taxus cuspidata, J. Nat. Prod., 2004, vol. 67, p. 58. https://doi.org/10.1021/np0301083
Zhang, C.H., Mei, X.G., Liu, L., and Yu, L.J., Enhanced paclitaxel production induced by the combination of elicitors in cell suspension cultures of Taxus chinensis, Biotechnol. Let., 2000, vol. 22, p. 1561. https://doi.org/10.1023/A:1005684901329
Khosroushahi, A.Y., Valizadeh, M., Ghasempour, A., Khosrowshahli, M., Naghdibadi, H., Dadpour, M.R., and Omidi, Y., Improved taxol production by combination of inducing factors in suspension cell culture of Taxus baccata, Cell Biol. Intern., 2006, vol. 30, p. 262. https://doi.org/10.1016/j.cellbi.2005.11.004
Bonfill, M., Bentebibel, S., Moyano, E., Palazon, J., Cusido, R.M., Eibl, R., and Pinol, M.T., Paclitaxel and baccatin III production induced by methyl jasmonate in free and immobilized cells of Taxus baccata, Biol. Plant., 2007, vol. 51, p. 647. https://doi.org/10.1007/s10535-007-0137-2
Onrubia, M., Moyano, E., Bonfill, M., Cusido, R.M., Goossens, A., and Palazon, J., Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate, J. Plant. Physiol., 2013, vol. 170, p. 211. https://doi.org/10.1016/j.jplph.2012.09.004
Furmanowa, M., Oledzka, H., Syklowska-Baranek, K., Jozefowicz, J., and Gieracka, S., Increased taxane accumulation in callus cultures of Taxus cuspidata and Taxus × media by some elicitors and precursors, Biotech. Let., 2000, vol. 22, p. 1449. https://doi.org/10.1023/A:1005611114628
Amirkavei Najafabadi, B., Qavami, N., Ebrahimi, M.A., Ebrahimi, P., and Zarinpanjeh, N., Enhancement of taxol production by applying amino acid complex along with chitosan in suspension culture of Taxus baccata L., J. Med. Plants, 2020, vol. 19, p. 99. https://doi.org/10.29252/jmp.19.76.99
Vidal-Limon, H.R., Almagro, L., Moyano, E., Palazon, J., Pedreno, M.A., and Cusido, R.M., Perfluorodecalins and hexenol as inducers of secondary metabolism in Taxus media and Vitis vinifera cell cultures, Front. Plant Sci., 2018, vol. 9, p. 335. https://doi.org/10.3389/fpls.2018.00335
Barrales-Cureno, H.J., Ramos Valdivia, A.C., and Soto Hernandez, M., Increased production of taxoids in suspension cultures of Taxus globosa after elicitation, Future Pharm., 2022, vol. 2, p. 45. https://doi.org/10.3390/futurepharmacol2010004
Veeresham, C., Mamatha, R., Prasad Babu, Ch., Srisilam, K., and Kokate, C.K., Production of taxol and its analogues from cell cultures of Taxus wallichiana, Pharm. Biol., 2003, vol. 41, p. 426. https://doi.org/10.1076/phbi.41.6.426.17822
Mirjalili, N. and Linden, J.C., Gas phase composition effects on suspension of Taxus cuspidata, Biotechnol Bioeng., 1995, vol. 48, p. 123. https://doi.org/10.1002/bit.260480206
Linden, J., Haigh, J.R., Mirjalili, N., and Phisaphalong, M., Gas concentration effects on secondary metabolite production by plant cell cultures, Adv. Biochem. Eng. Biotechnol., 2001, vol. 72, p. 27. https://doi.org/10.1007/3-540-45302-4_2
Kim, S.I., Choi, H.K., Kim, J.H., Lee, H.S., and Hong, S.S., Effect of osmotic pressure on paclitaxel production in suspension cell cultures of Taxus chinensis, Enzym. Microb. Technol., 2001, vol. 28, p. 202. https://doi.org/10.1016/s0141-0229(00)00292-1
Choi, H., Kim, S., Son, J., Hong, S., Lee, H., and Lee, H., Enhancement of paclitaxel production by temperature shift in suspension culture of Taxus chinensis, Enzyme Microb. Technol., 2000, vol. 27, p. 593. https://doi.org/10.1016/s0141-0229(00)00255-6
Zhong, J.J. and Yue, C.J., Plant cells: secondary metabolite heterogeneity and its manipulation, Adv. Biochem. Eng. Biotechnol., 2005, vol. 100, p. 53. https://doi.org/10.1007/b136412
ACKNOWLEDGMENTS
This work was performed within the framework of a state assignment given by the Ministry of Science and Higher Education of the Russian Federation (project nos. 121050500047‒5 and 121041200194‒7).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving humans and animals as subjects. The authors declare that they have no conflicts of interest.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by N. Balakshina
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tomilova, S.V., Globa, E.B., Demidova, E.V. et al. Secondary Metabolism in Taxus spp. Plant Cell Culture In Vitro. Russ J Plant Physiol 70, 23 (2023). https://doi.org/10.1134/S102144372270008X
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S102144372270008X