Abstract
Removal of aromatic compounds from fuels due to the strict regulations as well as carcinogenic and detrimental effects on the humans and the environment is one of the main steps in the petrochemical industry. Competitive hydrogenation of benzene in aromatic mixtures not only achieves this goal, but also prevents the significant reduction in the octane number by producing cyclohexane in the fuels. For this purpose, a series of nickel catalysts supported on the Ti-promoted mesoporous-carbon composites have been prepared by the wet impregnation method. Textural and physicochemical properties of catalysts were characterized using FT-IR, N2 adsorption-desorption, XRD, SEM, and EDAX. The activity of the powders was evaluated in the temperature range of 150–210°C. The results confirm that the Ni/Ti-SBA-15 catalyst shows the acceptable selectivity in the test temperature range and the highest activity at 190°C.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In recent decades, the reduction of the benzene concentration in the fuels has been of great interest due to its carcinogenic effects on humans and the destructive results on the environment. This is because of its incomplete combustion in engines and its release into the atmosphere [1–3]. However, it is essential to note that if benzene eliminated its harmful effects on health and the environment reduced. Still, octane number of the fuel and in other words, its combustion quality decreases. Competitive hydrogenation of benzene seems to be an efficient and economical method [4, 5]. In the method as mentioned above, benzene selectively hydrogenated in the mixture of aromatic and paraffinic substances. With this process, the carcinogenic and destructive effects of benzene reduce and also the production of the cyclohexane as a green and widely-used industrial solvent is available [6]. The point to consider is that the cyclohexane in the gasoline has the octane number close to benzene in the same mix [7]. Consequently, with selective hydrogenation of benzene to cyclohexane, the octane number of the fuel will not change significantly [8]. The mechanism of this process is not yet clear. However, based on the Eley–Rideal mechanism, hydrogen reacts molecularly with the adsorbed benzene [9]. Also, in the Horiuti–Polanyi model, the addition of hydrogen atoms is considered separately [10].
Among the several parameters that affected on hydrogenation conversion reaction temperature, the feed and hydrogen flow rates, support textural properties, the kind and loading percentage of metals have the critical roles [11]. Various intermediate metals reported as the active phase of catalysts in the hydrogenation reaction of benzene. The noble metal-base catalysts generally active at lower temperatures in comparison to non-precious metals (Ni, Co, and W) but the second group because of these easy-access and economic efficiency are preferred [2, 12]. Choose of nickel seems to be wise because of its acceptable performance due to its similar electronic structure to the platinum and the palladium, and cost-effectiveness [13–15]. Another factor affecting catalytic activity is the method of preparation. So far, various methods such as impregnation, ion exchange, precipitation-deposition, laser electrodispersion and hybrid impregnation-plasma have been reported for the preparation of nickel catalysts. Among them, the impregnation method is more frequently applied than other methods due to its cost-effectiveness, practical simplicity, and the possibility of loading metals in different quantities [16–18]. For structure-insensitive reactions such as hydrogenation, which kinetically controlled at low temperatures, and the number of available metal sites affects catalytic activity and selectivity, the dispersion of metal nanoparticles is important [19]. The textural properties and the high surface area of the supports make this possible.
Pie et al. [20] prepared RuNi–CNTs-x nano-composites via the galvanic replacement reaction method using carbon nanotube as a support and investigated the catalytic activity in benzene hydrogenation. The RuNi–CNTs-F catalyst showed the highest yield to cyclohexane up to 70.9%. Filippova et al. [21] evaluated the Dy–Ni/MS catalyst that synthesized by the template method in benzene and its derivatives hydrogenation reaction. The best conversion and selectivity of all aromatics were achieved at 170 and 120°C, respectively.
Given the importance and necessity of developing and optimizing active catalysts for the hydrogenation process, the main goal of this report was to design and prepare composites based on silicate and carbon materials that have not yet been used for the competitive hydrogenation of benzene process and simultaneously have the characteristics of silicate materials such as high surface area and the adjustable pore size and the characteristics of carbon adsorbents such as rich-pore structure, recycle-sufficiency, high thermal conductivity and surface area [5, 22]. The Ti-compounds have an active role in increasing catalytic activity due to the transfer of electron-proton [22].
Hexagonal Mesoporous Silica (HMS) is a silicate mesopore that is highly regarded as a catalytic support due to its high surface area, high thermal stability and ability to bond with organic and inorganic species due to the silanol groups present on the surface. There have been many reports of enhanced catalytic activity through the incorporation of heteroatoms such as titanium, gallium, and vanadium in the HMS structure [23]. SBA-15 (Santa Barbara Amorphous-15) includes regular and two-dimensional tubular channels. Compared to other regular structure mesopores, SBA-15 can be prepared with larger pores, which makes it more stable due to the greater thickness of the pores. Because the mesopores do not have very high catalytic activity in the pure state, replacing some of the structural silicones with Ti improves catalytic performance. Titania-promoted silica have the properties of silica and Titania, which makes them more active and stable. The results of studies in this field conforms that the addition of metals such as Ti in the structure of mesopores, affects their acidic behavior and increases the catalytic rate, especially in the early hours of the reaction [24]. Reports also indicate that the introduction of Titania into the structure of the catalytic supports increases the dispersion of the active metal phase on the catalytic surface [25].
On this basis, in the present study, Ni nanoparticles (NPs) loaded over the composite catalysts were synthesized and characterized. The purpose of this work was to investigate the changes in composites performance as a function of variables, especially the impact of type of the silicate mesopores (here SBA-15 and HMS) and their textural properties and morphology on the catalytic activity. The relation of characterization, properties and performance of the catalytic activity and selectivity of this reaction was explained.
EXPERIMENTAL
For the preparation of SBA-15 support, 4 g of polymer Pluronic 123 (P123) and 144 mL of hydrochloric acid (1.7 M) stirred at 40°C for 4 h. Subsequently, tetraethyl orthosilicate (TEOS) was added at a weight ratio of TEOS/P123 = 2, and the solution stirred at the same temperature for 2 h. The obtained gel then transferred to autoclave and subjected to hydrothermal reaction for 48 h at 100°C. The final product was also dried in an oven at 80°C for 12 h after filtration and washing with distilled water. The final powder calcined for 3 h at 540°C [26].
For the preparation of Ti–SBA-15–CNT composite (symbolized as T–S–C), in the initial step, after adding Р123 polymer and hydrochloric acid, the calculated amount of TiO2 (Si/Ti = 40) and Carbon Nano Tube (CNT) were added to the solution, and the rest of the steps was done as the instructions mentioned above.
For the preparation of HMS mesoporous, 1 g of ethanol and 1 g of silicate source (TEOS) stirred continuously for 30 min at 40°C (solution A). For the solution B, 1.2 g of dodecyl amine (DDA), 0.5 mL (1 M) hydrochloric acid, and 15 mL of distilled water rotated at room temperature for 5 min. In the final step, the two solutions mixed and stirred for 18 h to form the gel. The last gel calcined at 600°C for 6 h [6]. Ti–HMS–CNT composite (symbolized as T–H–C) is also obtained by adding a certain amount of TiO2 and Carbon Nano Tube (TEOS/CNT = 3.3) to the solution A and performing the other steps such as HMS synthesis. Multi-walled CNTs were purchased from US Research Nanomaterials, Inc. For purification, the substance refluxed with nitric acid (68%) for 5 h, then washed with distilled water and dried in the oven at 60°C.
To prepare 25% nickel catalysts, the calculated amount of Ni(NO3)2·6H2O added to each of the composites and stirred at 80°C until the formation of the gel. Then, dried at 100°C in the oven, and calcined for 4 h at 300°C.
Characterization tests of catalysts. Pore size and surface area of catalysts determined by the Brunauer–Emmett–Teller (BET) method (BELSORP MINI II). The abundance and distribution of Ni and Ti loaded over the prepared supports were measured by EDX-MAP analysis (AMETEK, model OCTANE PRIME). The crystal structure of the desired catalysts was examined by the X-ray diffraction method. These characterizations were monitored by X-PERT diffractometer with a 0.06° 2θ-step and 1 s per step in the scanning angle 2-theta (2θ) from 1° to 80°. Scanning electron microscope (SEM) images obtained by a HITACHI S-4160 instrument, those catalysts coated with gold and operating at an accelerating voltage of 30 kV. Gas chromatography model 7890, manufactured by the Ajilien Company (USA), equipped with the flame ion detector and a split/non-flush input chamber, used for the detection of products. This device has a BP5-type Moon Column (SGA Australia). BOMEM FT-IR spectrometer model Arid-Zone was used to identify and detect the functional groups of catalysts and the removal of residual surfactant from the dried samples in the 200–4000 cm–1 with a resolution of 4 cm–1 using KBr pellet.
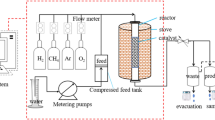
Catalyst evaluation. The selective hydrogenation of benzene performed in the continuous flow microreactor with fixed catalyst layer equipped with a thermocouple. This process is done using 0.2 g of each catalyst with inlet gas mixture (6 vol %. Benzene (Bz), 8 vol %. Toluene (Tu), and 8 vol %. Xylene (Xy), abbreviated as BTX, injection rate of 3 cc h–1 and in the 40 mL/min flow rate of hydrogen at the range of 150–210°C. The catalytic tests were carried out at various temperatures on the same catalyst. After the reduction of the catalyst under the hydrogen stream at 400°C for 2 h, the powders cooled to the desired temperatures. Benzene and the reaction products analyzed each 60 min using the Gas chromatography model 7890 with a flame ionization detector, operated at a programmed temperature. This device equipped with the BP5 capillary column (SGA Australia) containing 5% diphenyl and 95% dimethyl siloxane with a length of 30 m, a diameter of 0.25 cm, and a thickness of the stationary phase of 0.25 µm. The temperature of the injection chamber was set at 270°C and the detector at 300°C. The temperature program of the column was as follows: 3 min of stopping at 30°C, then increasing the temperature up to 80°C at a rate of 5°C/min, and then changing it at the rate of 30ºC/min until the temperature of 270°C. Nitrogen carrier gas (99.999%) and air were supplied by a nitrogen and aeration device model 6010 and hydrogen by the model 20H device of Perker Hanfin (England).
RESULTS AND DISCUSSION
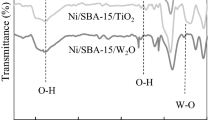
Physicochemical properties of the catalysts. Figure 1 shows the FTIR spectra of the mentioned Ni supported samples. In all spectra, the bands at around 1600 and 3400 cm–1 indicate the O–H stretching vibrations of physisorbed water [27]. The asymmetric stretching and bending vibration of ≡Si–O–Si≡ group in the HMS, and the SBA-15 are observed at 1100, 800 and 440 cm–1 [6, 28]. The absorption peak of carboxyl group stretching vibration and surface oxygen formation in CNT seen around 1710–1730 cm−1 [29]. Besides, the weak band that appears in 1580 cm–1 is related to the C–C vibration mode in CNT structure [30].
Figure 2 shows the X-ray diffraction of the Ni supported catalysts. The sharp peak that depicted at 2θ = 1.5° indexed to the hexagonal mesoporous structure of HMS. Also, the broad absorption peak appearing in the range of 2θ = 15°–30° indicates the amorphous structure of the silica (SiO2) phase in pure mesopores, Ti–HMS and Ti–SBA-15 structures [31, 32]. For Ni/S which is given as a reference for the SBA-15 as the catalytic support and Ni/T–S–C catalysts, the high-intensity diffraction that appears at 2θ = 1.1° demonstrates the mesoporous and ordered structures. The reflections of d100, d110, and d200 planes a[6]s well as hexagonal structure, appear with a broad and a weak peak in the range of 2θ = 1.9°–2.3° [33, 34]. The carbon nanotube characteristic peaks appear at angles 25.5°, 43°, and 53°, respectively, due to the reflection of C (002), C (100), and C (400) sheets in the hexagonal structure of graphite [35]. After the calcination of impregnated catalysts, by reducing the Ni catalysts under the hydrogen gas stream, the nickel phase (Ni2+) was formed. The nickel catalysts characterized by three main signals that appear at 2θ = 37.2°, 43.3° and 62.8°.
The surface and textural properties of the nickel catalysts, including the total pore volume, BET surface area, and pore diameter are summarized in Table 1. The high surface area is found 532 m2/g for the Ni/T–H–C catalyst. The volume of pores varies from 0.44 to 1.05 cm3/g. In addition, the pore volume of the Ni/T–S–C is less than the Ni/T–H–C composite, which may be due to the agglomeration of the Ti extra amount inside the pores. Surface properties of the Ni/HMS, including surface area, pore size, and etc., have been studied in detail in the previous work of this group [6].
The N2 adsorption-desorption isotherm diagrams, as well as the pore size distribution of prepared composites, shown in Figs. 3a and 3b. The Ni/T–S–C and the Ni/T–H–C present the type IV isotherm with the type H1 hysteresis loop, indicating the mesoporous nature of the composites and narrow cylindrical pores with the uniform shape and size. The pore size diagrams represent that the highest dispersion is in the range of 2–9 nm, which confirms the mesoporous structure of the composites. The surface morphology of the nickel catalysts obtained using the SEM analysis is presented in Figs. 4a–4d. Figures 4a and 4d show the HMS spherical-structure. Figures 4b and 4c illustrate well the cylinder-hexagonal structure of SBA-15. The rich hollow cylinder structure of the carbon nanotube is not seen, which seems to be due to the low content of this material compared to the silicate material. The prepared composites appear to have the different structure than each of the reference catalytic supports, and the pure catalytic supports are not clearly distinguishable because of the type of composite preparation method. Also, the aggregation of particles occurred through the impregnation of metals and finally calcination.
Activity results. The activity test results of the prepared catalysts were shown in Figs. 5a–5d. The activity of samples was evaluated in the temperature range of 150–210°C and at atmospheric pressure. These tests performed to investigate the composition effect of the supports on their catalytic performance in the competitive benzene hydrogenation reaction. The results of the Ni/HMS and the Ni/CNT catalysts were reported in previous papers of this group [6, 36]. The Ni/T–S–C catalyst performs best except for 210°C. It seems that the reason for the high activity of this composite is the high surface area for more dispersion of the active phase and their more comfortable access. At temperatures above 190°C, the saturation of the reactants over the catalyst surface appears to reduce the benzene conversion (Eq. (1)).
where CBz (%) is the benzene conversion in hydrogenation reaction.
The named-composite performs better than pure supports (SBA-15 and CNT). For the Ni/T–H–C composite, as the temperature rises to 190°C, the converting of the benzene to cyclohexane as the dominant product increases due to the increase in the vapor pressure of the reactants and easier access to them. However, at higher temperatures, the benzene conversion decreases. For catalysts, with increasing temperature, the selectivity for benzene hydrogenation and cyclohexane production in the BTX mixture increases (Eqs. (2), (3)).
where m and C are the molar ratio and conversion of benzene, toluene, or xylene, respectively. Cov (%) is the overall aromatics conversion in BTX mixture.
The highest hydrogenation of BTX belongs to the Ni/T–S–C catalyst at all temperatures. To evaluate the overall performance of catalysts, their yield was calculated using Eq. (4). The results confirm that the Ni/T–S–C composite has the most acceptable performance during the reaction. Figure 5d shows that increasing the conversion does not necessarily increase selectivity. Selectivity specifically related to the textural properties as well as the catalytic pore size.
where YCHE is the yield of cyclohexane and SBz is the benzene selectivity.
Kinetic study. The following Eq. (5) used to calculate the specific rates of catalysts. 0.2 g of catalysts reduced at 400°C before the reaction began. According to Table 2, the specific catalytic rate generally increases with increasing reaction temperature. It seems to be due to the ease of access to the reactants due to their increased partial pressure.
To calculate the activation energy of the catalysts, Arrhenius diagrams were plotted (not shown here), and its results were reported in Table 2. Those obtained results are less than the values reported in some articles for this reaction [1, 37]. The lowest amount of apparent activation energy related to the Ni/T–S–C. Activity test results confirm that this catalyst requires less energy than the rest to initiate the reaction.
These calculations carried out under identical conditions for all catalysts and conversion of less than 10% [1, 5, 37].
CONCLUSIONS
Competitive benzene hydrogenation in BTX mixture was performed using a series of silicate-carbon composites. The catalytic activities of these hybrids were evaluated as a function of temperature, as well as a composition effect in comparison to pure supports. Surface and physico-chemical properties of samples were characterized using FTIR, nitrogen adsorption-desorption XRD, SEM, and EDAX. The results of the activity tests show that the Ni/T–S–C composite reacts best for benzene conversion (except for temperature 210°C) and selectivity to produce cyclohexane. The lowest activation energy value obtained for this catalyst. In general, composition plays an effective role in promoting catalytic activity.
Change history
30 December 2022
Corrected PDF Copyright
25 January 2023
An Erratum to this paper has been published: https://doi.org/10.1134/S0965544122100188
REFERENCES
Wojcieszak, R., Monteverdi, S., Mercy, M., Nowak, I., Ziolek, M., and Bettahar, M.M., Appl. Catal. A: Gen., 2004, vol. 268, pp. 241–253. https://doi.org/10.1016/j.apcata.2004.03.047
Cooper, B.H. and Donnis, B.B.L., Appl. Catal. A: Gen., 1996, vol. 137, pp. 203–223. https://doi.org/10.1016/0926-860X(95)00258-8
Barrio, V.I., Arias, P.L., Cambra, J.F., Güemez, M.B., Pawelec, B., and Fierro, J.L.G., Appl. Catal. A: Gen., 2003, vol. 242, pp. 17–30. https://doi.org/10.1016/S0926-860X(02)00489-1
Peyrovi, M., Rostamikia, T., and Parsafard, N., Energy Fuels, 2018, vol. 32, pp. 11432–11439. https://doi.org/10.1021/acs.energyfuels.8b02952
Mohammadian, Z., Peyrovi, M.H., and Parsafard, N., ChemistrySelect, 2018, vol. 3, pp. 12639–12644. https://doi.org/10.1002/slct.201802760
Peyrovi, M.H., Parsafard, N., and Mohammadian, Z., Chin. J. Chem. Eng., 2018, vol. 26, pp. 521–528. https://doi.org/10.1016/j.cjche.2017.05.022
Balaban, A., Kier, L., and Joshi, N., MATCH Commun. Math. Comput. Chem., 1992, vol. 28, pp. 13–27.
Mohammadian, Z., Peyrovi, M.H., and Parsafard, N., Silicon, 2020, vol. 12, pp. 1943-1948. https://doi.org/10.1007/s12633-019-00302-6
Kehoe, J. and Butt, J., J. Appl. Chem. Biotechnol., 1972, vol. 22, pp. 23–30. https://doi.org/10.1002/jctb.2720220105
Horiuti, I. and Polanyi, M., Trans. Faraday Soc., 1934, vol. 30, pp. 1164–1172. https://doi.org/10.1039/TF9343001164
Mohammadian, Z., Peyrovi, M.H., and Parsafard, N., ChemistrySelect, 2019, vol. 4, pp. 11116–11120. https://doi.org/10.1002/slct.201901780
Stanislaus, A. and Cooper, B.H., Catal. Rev. Sci. Eng., 1994, vol. 36, pp. 75–123. https://doi.org/10.1080/01614949408013921
Vogt, C., Groeneveld, E., Kamsma, G., Nachtegaal, M., Lu, L., Kiely, C.J., Berben, P.H., Meirer, F., and Weckhuysen, B.M., Nat. Catal., 2018, vol. 1, pp. 127–134. https://doi.org/10.1038/s41929-017-0016-y
Younas, M., Loong Kong, L., Bashir, M.J., Nadeem, H., Shehzad, A., and Sethupathi, S., Energy Fuels, 2016, vol. 30, pp. 8815–8831. https://doi.org/10.1021/acs.energyfuels.6b01723
Mashkovsky, I.S., Baeva, G.N., Stakheev, A.Y., Voskoboynikov, T.V., and Barger, P.T., Mendeleev Commun., 2009, vol. 2, pp. 108–109. https://doi.org/10.1016/j.mencom.2009.03.020
Jiménez-González, C., Boukha, Z., de Rivas, B., González-Velasco, J.R., Gutiérrez-Ortiz, J.I., and López-Fonseca, R., Energy Fuels, 2014, vol. 28, pp. 7109–7121. https://doi.org/10.1021/ef501612y
Kavalerskaya, N.E., Lokteva, E.S., Rostovshchikova, T.N., Golubina, E.V., and Maslakov, K.I., Kinet. Catal., 2013, vol. 54, pp. 597–606. https://doi.org/10.1134/S0023158413050066
Chang, F.-W., Tsay, M.-T., and Kuo, M.-S., Thermochim. Acta, 2002, vol. 386, pp. 161–172. https://doi.org/10.1016/S0040-6031(01)00771-7
Beierlein, D., Häussermann, D., Pfeifer, M., Schwarz, T., Stöwe, K., Traa, Y., and Klemm, E., Appl. Catal. B, 2019, vol. 247, pp. 200–219. https://doi.org/10.1016/j.apcatb.2018.12.064
Pei, A., Ruan, L., Zeng, P., Fu, H., Zeng, L., Liu, J., Zhang, H., Yang, K., Zhu, L., and Chen, B.H., Catal. Lett., 2021, vol. 151, pp. 773–786. https://doi.org/10.1007/s10562-020-03341-6
Filippova, E.O., Tokranov, A.A., Shafigulin, R.V., and Bulanova, A.V., Russ. J. Appl. Chem., 2020, vol. 93, pp. 741–747. https://doi.org/10.1134/S1070427220050158
Su, J. and Chen, J.-S., Microporous Mesoporous Mater., 2017, vol. 237, pp. 246–259. https://doi.org/10.1016/j.micromeso.2016.09.039
Kruk, M., Jaroniec, M., and Sayari, A., Microporous Mater,, 1997, vol. 9, pp. 173–182. https://doi.org/10.1016/S0927-6513(96)00099-5
Ye, W., Lin, Z., Dong, B., Kang, J., Zheng, X., and Wang, X., Mater. Sci. Appl., 2011, vol. 2, p. 661. https://doi.org/10.4236/msa.2011.26091
Zepeda, T.A., Halachev, T., Pawelec, B., Nava, R., Klimova, T., Fuentes, G.A., and Fierro, J.L.G., Catal. Commun., 2006, vol. 7, no. 1, pp. 33–41. https://doi.org/10.1016/j.catcom.2005.07.021
Fulvio, P.F., Pikus, S., and Jaroniec, M., J. Colloid Interface Sci., 2005, vol. 287, pp. 717–720. https://doi.org/10.1016/j.jcis.2005.02.045
Parsafard, N., Peyrovi, M.H., and Hajiabadi, M.A., Phys. Chem. Res., 2020, vol. 8, pp. 203–213. https://doi.org/10.22036/PCR.2020.206012.1694
Mohammadian, Z., Peyrovi, M.H., and Parsafard, N., Reac. Kinet. Mech. Cat., 2019, vol. 127, pp. 979–990. https://doi.org/10.1007/s11144-019-01611-y
Salam, M.A. and Burk, R., Arab. J. Chem., 2017, vol. 10, pp. S921–S927. https://doi.org/10.1016/j.arabjc.2012.12.028
Zhou, J.-H., Sui, Z.-J., Zhu, J., Li, P., Chen, D., Dai, Y.-C., and Yuan, W.-K., Carbon, 2007, vol. 45, pp. 785–796. https://doi.org/10.1016/j.carbon.2006.11.019
Lin, X., Zeng, X., Zhou, R., and Wang, H., Reac. Kinet. Mech. Cat., 2019, vol. 126, pp. 353–364. https://doi.org/10.1007/s11144-018-1520-z
Bérubé, F., Khadhraoui, A., Janicke, M.T., Kleitz, F., and Kaliaguine, S., Ind. Eng. Chem. Res., 2010, vol. 49, pp. 6977–6985. https://doi.org/10.1021/ie901659k
Qian, X.F., Kamegawa, T., Mori, K., Li, H.X., and Yamashita, H., J. Phys. Chem. C, 2013, vol. 117, pp. 19544–19551. https://doi.org/10.1021/jp4071373
Devi, P., Das, U., and Dalai, A.K., Chem. Eng. J., 2018, vol. 346, pp. 477–488. https://doi.org/10.1016/j.cej.2018.04.030
Asl, M.S., Farahbakhsh, I., and Nayebi, B., Ceramics Int., 2016, vol. 42, pp. 1950–1958. https://doi.org/10.1016/j.ceramint.2015.09.165
Mohammadian, Z., Peyrovi, M.H., Parsafard, N., Chem. Phys. Lett., 2019, vol. 715, pp. 367–374. https://doi.org/10.1016/j.cplett.2018.12.003
Pushkarev, V.V., An, K., Alayoglu, S., Beaumont, S.K., and Somorjai, G.A., J. Catal., 2012, vol. 292, pp. 64–72. https://doi.org/10.1016/j.jcat.2012.04.022
ACKNOWLEDGMENTS
The authors thank the National Iranian Oil Refining & Distribution Company (NIORDC) for financial support of this project (D-98-002) and also from Shahid Beheshti University due to its facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
The original online version of this article was revised: Due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammadian, Z., Parsafard, N. & Peyrovi, M.H. Role of Supports in Ni Nanoparticles Ti-Promoted Composite Catalysts for the Total Hydrogenation of Benzene. Pet. Chem. 62, 1072–1080 (2022). https://doi.org/10.1134/S0965544122090055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544122090055