Abstract—
In the experiment, plasma–dust clouds were obtained from the substance of the Tsarev meteorite, a simulant of lunar regolith LMS-1D and ilmenite concentrate using a microwave discharge in powder media. For each of the samples, the dynamics of the development of the discharge and the formation of a plasma–dust cloud with subsequent relaxation after the end of the microwave pulse were recorded. From the emission spectra of the plasma and the surface of a solid body, the temperatures of the gas, electrons and surface were determined. A comparison of the phase and elemental composition of the initial samples and samples after exposure to plasma showed that there is no significant change in the composition. However, scanning electron microscopy results clearly indicate spheroidization of the original angular and irregularly shaped particles. The appearance of spherical particles is also observed, the dimensions of which are larger than the linear dimensions of the particles in the original sample. The results obtained indicate the possibility of using such experiments to study chemical and plasma-chemical processes of synthesis and modification of substances under conditions of plasma–dust clouds encountered in space phenomena.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
During various cosmic processes, a multiphase plasma–dust environment is formed (Kuznetsov et al., 2023; Popel et al., 2018; Zakharov et al., 2020; Johnstone, 2013; Goetz et al., 2022). Such plasma–dust clouds can contain not only plasma, but also dust particles, melt drops, and neutral gas. Impacts of meteoroids and micrometeoroids on the surface of an astronomical body (Gerasimov et al., 1998; Popel et al., 2020), the movement of meteoroids in the atmosphere of planets (Bronshten, 1981; Silber et al., 2018) are processes in which such a multiphase environment is formed.
Existing ways of laboratory modeling suggest direct methods (Mocker et al., 2011; Friichtenicht, 1962; Shu et al., 2012; Semkin et al., 2007, 2009; Hudepohl et al., 1989; Best and Rose, 1999; Igenbergs et al., 1987; Vricella et al., 2017; Chhabildas et al., 1995; Thornhill et al., 2006; Lexow et al., 2013) modeling and “indirect” (Helber et al., 2019; Loehle et al., 2017; Blanchard and Cunningham, 1974; Bones et al., 2016; Gómez Martín et al., 2017; Sorokin et al., 2020a; Stöffler and Langenhorst, 1994; Fulvio et al., 2021; Moroz et al., 1996; Grokhovsky et al., 2020; Bezaeva et al., 2010). Direct modeling methods are based on accelerating the impactor to velocities characteristic of meteoroids of the Solar System, and in indirect methods, certain stages of processes or conditions are reproduced.
The creation of plasma–dust clouds from the substance of meteorites, regoliths, or equivalent artificial mixtures in laboratory conditions makes it possible to study certain issues of space weathering (Prince et al., 2020; Sasaki et al., 2003; Sorokin et al., 2020b), prebiological synthesis (Managadze, 2003; Zaitsev et al., 2016; Ferus et al., 2020; Martins et al., 2013). The synthesis of organic substances from the simplest inorganic substances under conditions of a plasma–dust cloud differs from other possible ways of prebiological synthesis by the presence of a large number of possible plasma-chemical reactions (with atoms and molecules in an excited state, with charged particles, photolytic reactions under the influence of ultraviolet radiation), as well as the presence of particles of dust, which can play the role of both crystallization centers and reaction catalysts (Parmon, 1999; Gerasimov and Safonova, 2008; Peters et al., 2023; Saladino et al., 2013, 2018; Snytnikov, 2002, 2010).
Charged dust is present everywhere in space and the Earth’s atmosphere: in interstellar matter, nebulae and clouds, supernova remnants, protoplanetary disks, in the vicinity of active galactic nuclei, in the Earth’s ionosphere and meteoroid tails, in the tails of comets, in the atmospheres of other planets and in the exospheres of atmosphereless bodies (satellites and asteroids). Organic compounds can be found on individual bodies and dust particles.
Complex chemical reactions could occur on dust particles in the molecular layers of protoplanetary disks and organic compounds could be formed, including simple amino acids (for example, glycine). One can expect the development of processes of synthesis of organic compounds in the transition regions of protoplanetary disks, where the concentrations of charged particles (including charged dust) are still high, but at the same time dense molecular regions are present. Also, the prerequisites for the development of these processes may be stellar flares, which produce high radiation passing into denser layers and precipitation of charges. As a result, there are areas nearby with oppositely charged particles, ions and radical ions, which are attracted and enter into chemical reactions more intensely.
In small meteorites (or in the upper parts of the cometary core), in which internal rocks have not been heated, compounds can exist in the form in which they were deposited on the surface of dust grains during the formation of planets in the protoplanetary disk or formed on the surface of dust as a result of chemical reactions. This is confirmed by observations. During the 2001 Leonid meteor shower, data collected by the Leonid MAC showed that meteoroids ranging in size from 50 µm to 10 cm were rich in CN compounds (Jenniskens et al., 2004) as well as H2, O, H, H2O, CO2, CO, CH4, CH, C2HO (Jenniskens, 2001). O and N2 bands were observed in the spectra of meteor showers in different excited states.
Comets are a source of meteoroids that can enter the Earth’s atmosphere and serve as triggers for the prebiological synthesis of organic substances and the origin of life. Comets and the small meteoroid bodies they generated hardly experienced any thermal metamorphism, and their composition could have preserved more complex substances synthesized on dust particles during the formation of planets (Vasyunin et al., 2008). When a meteoroid passes through the Earth’s atmosphere, high temperatures and concentrations of particles are created in the plasma–dust tail of the meteoroid, which turns out to be favorable conditions for the rapid occurrence of a number of chemical reactions. At the same time, rapid thermal “hardening” of synthesis products can favor the preservation of synthesized organic substances.
It is known that nitrogen is an essential element of many organic compounds necessary for the life of terrestrial organisms. Such compounds are nitrogenous bases (building blocks of nucleic acids and ATP, etc.) and amino acids (structural elements of peptides, proteins, etc.). At the same time, nitrogen gas (the predominant component of the Earth’s atmosphere) is a rather chemically inert substance. But the converted kinetic energy of the meteoroid is enough so that during the interaction of atmospheric gases and vapors (N2, CO2, H2O) with simple and complex substances, as well as individual functional groups of substances present in meteorites (C, N, H, CN, H2O, etc.), such compounds as HCN, NO were obtained, under conditions of high temperatures—nitrogenous bases, sulfur- and fluorine-containing compounds, etc.
In our studies on the synthesis of micro- and nanoparticles in a microwave discharge in metal/dielectric powder mixtures (Gayanova et al., 2023; Skvortsova et al., 2021a, 2023a; Batanov et al., 2013; Akhmadullina et al., 2019; Sokolov et al., 2023) we obtain plasma–dust clouds even under atmospheric pressure of the working gas. We also used this experimental setup to create a cloud of particles from powder samples that replicate the chemical composition of lunar regolith (Skvortsova et al., 2017, 2019, 2021b, 2023b; Batanov et al., 2022; Kachmar et al., 2021). In a cloud containing plasma, evaporated matter, melt drops, dust particles, the gas temperature is about 5000 K, and the plasma density is ~1014 cm–3. During plasma exposure, new substances and phases are formed, and morphological changes in dust particles occur. In this case, the heating process is fast (<50 μs), since it is associated with the development of a low-threshold discharge at the metal-dielectric or semiconductor–dielectric contacts (Batanov et al., 2004; Batanov and Kossyi, 2015), and not with the direct heating of substances in a microwave field (Bykov et al., 2001; Vodop’yanov et al., 2017; Rybakov et al., 2006, 2013; Rybakov and Buyanova, 2018; Egorov et al., 2010). Direct heating of substances in a microwave field is a relatively slow process, even taking into account the possible development of thermal instability. The probability of initiating microwave breakdown in mixtures of powder samples depends on the proximity to the optimum size of conductive particles (corresponding to the thickness of the skin layer), the proportion of conductive particles in the mixture, and the intensity of microwave radiation.
When used in experiments on microwave discharge in mixtures of powders as initial mixtures of mineralogical samples characteristic of cosmic bodies, it becomes possible to simulate plasma–dust environments formed, for example, when a micrometeoroid hits the surface of an atmosphereless body or when a meteoroid moves in the atmosphere of a planet. Moreover, such modeling will make it possible to recreate a multiphase environment with its characteristic processes of transformation and synthesis of substances.
In the present work, we used three types of samples in experiments as starting substances: (1) ilmenite concentrate, since ilmenite is often a phase contained in lunar regolith; (2) lunar dust simulant LMS-1D (Lunar mare simulant—dust) (Long-Fox and Britt, 2023; Madison et al., 2022; Stockstill-Cahill et al., 2021, 2022), as a sample that replicates the mineralogical composition and the distribution of particles by size in the regolith of the lunar seas (Florensky et al., 1975; Slyuta, 2014; Heiken et al., 1991; NASA Lunar sample and photocatalog; Lunnyi grunt..., 1974, 1980; Barsukov and Surkov, 1979) or more precisely, its finest fraction is lunar dust (Kuznetsov et al., 2016; Zakharov et al., 2020; Park et al., 2006); (3) the substance of the Tsarev meteorite (ordinary chondrite L5) (Semenova et al., 1984; Migdisova et al., 1982; Barsukova et al., 1982; Slyuta, 2017), as an example of an original substance of cosmic nature with a complex mineralogical composition. Separately, we have already presented the main results of experiments with the substance of the Tsarev meteorite in (Akhmadullina et al., 2023).
EXPERIMENTAL CONDITIONS
As a source of linearly polarized microwave radiation with a wavelength λ = 4 mm (frequency f = 75 GHz) a gyrotron is used (Litvak et al., 2021). The experiments use pulses with a duration of up to 10 ms and a power of up to 450 kW. Microwave radiation at the gyrotron output is a Gaussian beam. This beam from the gyrotron to the sample is transmitted through a quasi-optical system of focusing mirrors (Fig. 1). At the sample location, the diameter of the microwave beam is 5 cm, which provides average electromagnetic wave intensities of up to 20 kW/cm2.
Experiment scheme: (1) gyrotron, (2) focusing mirror of the quasi-optical path, (3) flat mirror, (4) high-speed camera Phantom VEO 710L, (5) quasi-optical microwave coupler, (6–8) detectors of incident, reflected and transmitted microwave radiation, (9) plasma-chemical reactor, (10) end lenses of transmitting light guides of Avantes AvaSpec spectrometers.
The powder sample under study is placed horizontally in a thin layer (1–2 mm) in the form of a spot with a diameter of 4–5 cm on the surface of a quartz disk with a diameter of 8 cm. To limit the scattering and collection of the substance during the experiment, it is placed vertically on a quartz disk (6–8 mm thick) and a thin-walled quartz tube with a diameter of 7 cm and a height of up to 10 cm is installed coaxially with it. The test sample, assembled with a quartz disk and a quartz tube, is placed in a vacuum chamber (up to 10–2 Torr) plasma chemical reactor (Fig. 1) so that the center of the powder sample coincides with the axis of the microwave beam. The plasma-chemical reactor has quartz windows transparent to microwave radiation at the bottom and top. Microwave radiation is supplied to the plasma–chemical reactor from the bottom up, i.e., passes through the powder layer, and the discharge will occur on the upper open surface of the powder sample. This is possible because the initial (under normal conditions) absorption coefficient of microwave radiation does not exceed 30%.
To estimate the magnitude and dynamics of the absorption coefficient of microwave radiation in a powder sample and a plasma–dust cloud, a system of balanced microwave measurements is used (Fig. 1). With a time resolution of 200 ns, the level of supplied microwave power, the level of reflected radiation (the sum of reflections from stationary objects and the plasma–dust cloud) and the level of radiation passed through the powder sample and the plasma–dust cloud are recorded.
Registration of emission spectra of plasma and surfaces of solid bodies makes it possible to determine: electron temperature Te (by relative intensity of metal lines); rotational gas temperature Tr (according to the bands of the TiO molecule), which is as close as possible to the translational temperature under microwave discharge conditions; solid surface temperature Ts (along the radiation continuum in the Wien region of the spectrum), which corresponds to the surface temperature of the powder sample. The lines of sight of the spectrometers have three characteristic directions (Fig. 1): from above, the beam passes through the plasma–dust cloud and rests on the upper surface of the powder sample; from the side, the beam passes through the plasma–dust cloud parallel to the surface of the powder sample; from below, the beam passes through the entire layer of the powder sample. In total, several spectrometers with different swaths and spectral resolutions are used in the studies (AvaSpec-3648-U80: range 371–920 nm, resolution 0.36 nm; AvaSpec-ULS4096-CL-2-EVO 2109527U2: range 219–381 nm, resolution ~0.1 nm: 2109528U2: range 379–521 nm, resolution ~0.1 nm; AvaSpec-ULS2048CL-EVO 210952U1: range 520–739 nm, resolution ~0.25 nm) (Sokolov et al., 2023).
The dynamics of the processes of development of a microwave discharge and the evolution of a plasma–dust cloud are recorded by a high-speed video camera Phantom VEO 710L (monochrome, 1280 × 800 pixels, 7400 fps, 680 000 fps with a resolution of 64 × 8 pixels, minimum exposure time 1 μs, ISO sensor sensitivity Mono 6400D, dynamic range 12 bits), which is located above the plasma–chemical reactor (Fig. 1).
DESCRIPTION OF SAMPLES
Ilmenite Concentrate
A commercially available ilmenite concentrate was purchased in the form of a powder with a particle size of no more than 100 μm. The documentation provided by the supplier indicates the mineral composition (ilmenite 95%, rutile 3%, zircon 0.4%) and elemental composition (Table 1), obtained using X-ray fluorescence analysis. The ilmenite concentrate was ground by grinding in an agate mortar for 10 min, and 1 g of the material was used as a sample in the experiment without adding additional metal powder. As mentioned above, low-threshold microwave breakdown occurs in a mixture of metal and dielectric powders (usually a metal mass fraction of 5% is required), or a semiconductor and dielectric. Ilmenite has semiconductor properties, so we initially assumed that a microwave discharge could develop in it without additives. For the prepared ilmenite sample, micrographs were obtained in Figs. 2 and 3. The measurements were carried out using a Zeiss Merlin scanning electron microscope (SEM) equipped with an Oxford Instruments INCAx-act X-ray microanalysis attachment. An accelerating voltage of 10 kV was used to obtain images, which made it possible to simultaneously obtain sufficiently high-quality images of materials and carry out measurements using X-ray energy-dispersive spectroscopy (EDS). The elemental composition of the analyzed areas generally corresponds to that declared by the supplier. The particles have an irregular shape, in some cases with prominent sharp edges, the particle size reaches 100 μm.
Lunar Dust Simulant LMS-1D
The LMS-1D lunar dust simulant used in the experiment was produced by ExolithLab (United States). The simulant replicates the mineralogical composition (Table 2), elemental composition (Table 3) and particle size distribution of the dust of the lunar seas. Elemental composition in Table 3 was obtained using XRF analysis by the manufacturer. The composition and particle size distributions are provided by the manufacturer. The particle size range is 0.04–32 µm with a median size of 4 µm. When preparing the sample for the experiment, 10% (by weight) of metallic magnesium (particle size no more than 75 μm) was added to LMS-1D. Such an additive is necessary to initiate microwave breakdown, since lunar regolith, and therefore its simulant, is mainly a composition of dielectric materials, without inclusions of neutral metals. It is worth noting that nanophase iron (Taylor et al., 2001; Basu, 2005), which is formed in lunar dust, in particular, by thermal reduction during the passage of a shock wave during micrometeorite impacts (Sorokin et al., 2020b), is absent in the simulant used. And nanophase iron is a characteristic product of space weathering of lunar regolith. Its presence could lead to the occurrence of a low-threshold microwave breakdown in the simulant without the need to add metal powder. After preparing a mixture of LMS-1Dc 10% magnesium metal powder, the resulting sample was ground in a mortar to homogenize and break up agglomerates. We obtained SEM microphotographs of several areas (Fig. 4), superimposed on them with multilayer element distribution maps and individual element distribution maps (Fig. 5). The elemental composition was within the limits stated by the manufacturer. Added particles of neutral magnesium are reliably detected in micrographs. The particles of the lunar dust simulant have an irregular shape, in some cases with prominent sharp edges; the particle size reaches 100 μm. Small-sized particles predominantly form agglomerates and also cover the surface of larger particles, which makes it difficult to identify and describe the shape of the latter. The sample does not contain spherical glass particles, the presence of which is characteristic of lunar regolith (Slyu-ta, 2014; Popel et al., 2020).
Substance of the Tsarev Meteorite
The mineralogical and elemental composition of the Tsarev meteorite, which is a chondrite of petrographic type L5, was studied previously (Semenova et al., 1984; Migdisova et al., 1982; Barsukova et al., 1982; Oshtrakh et al., 2008, 2013a, 2013b; Maksimova et al., 2014, 2017, 2018). The main phases are olivine, orthopyroxene, troilite, clinopyroxene, the metal phase is represented mainly by an alloy of iron, nickel and cobalt.
The 102-g sample we were provided with was precleaved into several pieces which were then ground in a Fritsch Pulverisette 7 ball mill (in a tungsten carbide beaker using tungsten carbide balls). Grinding was carried out at 600 rpm 2 times for 10 min.
The particle size of the meteorite substance after grinding, measured using the dynamic light scattering method, lies in the range of 0.2–19.7 μm. SEM microphotographs (Fig. 6) generally confirm these dimensions. The particles are irregular in shape, in some cases with prominent sharp edges. No spherical glass particles were found in the sample. Larger particles are mainly agglomerates of smaller particles.
In samples of the substance examined with SEM and XRD, no individual particles of iron or other metal were found. Element distribution maps are presented in Fig. 7, and the elemental composition for a separate sample is presented in Table 4. The composition was within the expected limits; however, it was not possible to determine the proportion of cobalt in the sample within the error limits of the method. The presence of nickel and high iron content indicate that, although individual metal particles cannot be detected in micrographs, iron–nickel alloys may be present in the sample. This gave us the opportunity to assume that the substance of the Tsarev meteorite itself will be sufficient to initiate microwave breakdown, and the addition of additional metal powder will not be required.
Our studies of the phase composition of a sample of meteorite material in (Akhmadullina et al., 2023) revealed only the main phases in the form of olivine and orthopyroxene. The detection of other phases by X-ray phase analysis (XRD) is hampered by their small quantity (content less than 3% by volume) and low crystallinity for a number of phases.
EXPERIMENTAL RESULTS
Ilmenite Concentrate
The working gas in the experiment was atmospheric-pressure air. The microwave radiation power was 300–400 kW, and the pulse duration was 6–8 ms. The sample was exposed to five microwave pulses with a total energy of 11.4 kJ. The discharge in the powder sample of the ilmenite concentrate was initiated almost immediately at the front (~100 μs) of the microwave pulse. Photographs of the sample before and after exposure to the discharge are shown in Fig. 8.
Photos of samples placed on a quartz substrate inside a quartz tube: (a) ilmenite concentrate before exposure to the discharge; (b) LMS-1D lunar dust simulant with the addition of metallic magnesium powder before exposure to the discharge; (c) substance of the Tsarev meteorite before the impact of the discharge; (d) ilmenite concentrate after exposure to the discharge; (e) LMS-1D lunar dust simulant with the addition of metallic magnesium powder after exposure to a discharge; (f) substance of the Tsarev meteorite after exposure to the discharge. Color figure is available in the electronic version of the article.
The development of a discharge in a powder sample is accompanied by a radial expansion of the plasma formation above the sample to dimensions on the order of the width of the microwave beam. This is a longer process (0.5–1 ms) compared to the primary breakdown. During the microwave pulse, the plasma–dust formation has the highest temperature and glow intensity (Fig. 9, frame at 5.35 ms); the scattering of particles into the reactor volume is visually observed. After the end of the microwave pulse (Fig. 9, frame at 18.3 ms), the glow intensity of the plasma–dust formation begins to decrease. Under conditions of atmospheric pressure of the working gas, the plasma concentration existing during the microwave pulse (~1014 cm–3, was previously measured from the Stark broadening of the hydrogen line Hα in experiments with the addition of a small percentage of hydrogen-containing impurities) should decrease by several orders of magnitude over a time of about 1–2 ms (Reiser, 2009). Therefore, the high-speed camera frame at 18.3 ms (Fig. 9) already corresponds to a gas and dust cloud, and the glow is caused by thermal radiation of heated gas and individual dust particles.
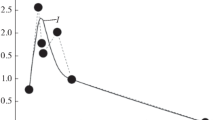
In the emission spectra (Fig. 10) recorded by a survey spectrometer (Avantes AvaSpec-3648-U80, spectral range 371–920 nm, resolution 0.36 nm), the line of sight of which is directed from bottom to top and passes through the entire layer of the powder sample, lines of neutral iron Fe I, neutral titanium Ti I, titanium ion Ti II, molecular bands TiO, FeO, as well as impurity lines (Na, Li, K). This spectrum makes it possible to determine the temperature of thermal radiation along the continuum in the Wien region of the spectrum (Fig. 11) (Voronova et al., 2021). It is obvious that thermal radiation with such a line of sight will be dominated by radiation from a solid body, rather than from plasma or gas in a plasma–dust cloud. Since the energy in a pulse of microwave radiation (up to 3 kJ) is fundamentally not enough to heat the entire mass (1 g) of the sample to experimentally measured temperatures of 1500–3000 K, this temperature characterizes the thin surface layer in which the main interaction of sample particles occurs with plasma and microwave radiation.
The rotational temperature of the gas component of the plasma–dust cloud was determined from the γ‑TiO molecular band (Hermann et al., 2001) in the spectrum obtained with the AvaSpec-ULS2048CL-EVO spectrometer (210952U1: range 520–739 nm, resolution ~0.25 nm), which has the best resolution, and whose line of sight passed parallel to the surface of the powder sample. The calculation of rotational temperatures is hampered by the presence of a thermal continuum, so the calculated value has a significant error Tr = 5500 ± 1500 K.
More detailed spectra were taken with an AvaSpec-ULS4096-CL-2-EVO spectrometer (2109527U2: range 219–381 nm, spectral resolution ~0.1 nm; 2109528U2: range 379–521 nm, spectral resolution ~0.1 nm). The electron temperature was calculated from the relative intensity of pairs of metal lines (two pairs of Fe I lines 396.23 and 407.15 nm, 396.23 and 438.35 nm) under the assumption that there is an equilibrium between the population of some electronic levels of elements in the gas phase and the temperature of the electron gas (Lochte-Holtgreven, 1971), using
where I is the line intensity, A is the transition probability, g is the degeneration coefficient, λ is the wavelength, E is the energy of the upper transition level, T is the temperature, k is the Boltzmann constant. Indices 1 and 2 refer to two different lines of the same element.
The resulting temperature of 5000 K turns out to be less than the average value of the rotational temperature, which may indicate some errors in the assumptions of the calculation methods used or uneven temperature distribution in the plasma–dust cloud.
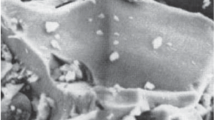
To obtain SEM microphotographs (Figs. 12, 13) and maps of the distribution of elements (Figs. 14, 15) by XRD, samples of the ilmenite concentrate substance after exposure to a microwave discharge were taken both from the bulk of the substance on a quartz disk (Fig. 12), and from the inner walls of the quartz tube (Fig. 13). In the sample from the bulk of the substance, particles with an angular shape are predominant, as in the original sample, but a significant number of spherical particles also appeared. Particle spheroidization is a characteristic process of plasma exposure (Samokhin et al., 2019; Károly and Szépvölgyi, 2005). Spherical particles do not have a smooth surface and a porous structure. A number of smaller (~1 µm) particles settled on large particles (>10 µm). Judging by the element distribution maps (Figs. 14, 15), there was no noticeable change in the elemental composition compared to the original sample. Even spherical particles do not have a different distribution of elements from other particles. Local measurements of elemental composition (Table 5) show some scatter in the content of Fe, Ti and O.
Multilayer maps of the distribution of elements in samples of ilmenite concentrate after exposure to a microwave discharge: on the left—a sample from the bulk of the sample; on the right is a sample from the inner walls of a quartz tube. Color figure is available in the electronic version of the article.
Lunar Dust Simulant LMS-1D
The working gas in the experiment was argon at atmospheric pressure. The microwave radiation power was 400 kW and the pulse duration was 6 ms. The sample was exposed to five microwave pulses with a total energy of 7.2 kJ. A discharge in a powder sample of the lunar dust simulant LMS-1D (with the addition of a 10% mass fraction of metallic magnesium powder) was initiated at the front of a microwave pulse. Photographs of the sample before and after exposure to the discharge are shown in Fig. 8. The dynamics of discharge development in a powder sample of a lunar dust simulant is hardly any different from a sample of ilmenite concentrate: at the initial stage, radial expansion of the plasma formation occurs; the highest radiation intensity and temperature of the plasma–dust formation are observed during the microwave pulse (Fig. 16, frame at 5.35 ms), when the scattering of particles into the reactor volume is also visually observed; after the end of the microwave pulse (Fig. 16, frame at 18.3 ms), the intensity of the glow of the plasma–dust formation begins to decrease, as do the temperatures.
Footage of the development of a microwave discharge in a sample of the lunar dust simulant LMS-1D. The frame start time (milliseconds) from the moment of breakdown is indicated on each frame in the upper left corner. The frame exposure time is 100 μs. Microwave power 400 kW, microwave pulse duration 6 ms.
In the emission spectra (Fig. 17), recorded with a survey spectrometer, the line of sight of which passes from bottom to top through the entire layer of the powder sample, lines of neutral iron Fe I, titanium Ti I, potassium K I and its regular companion rubidium Rb I, sodium Na I, iron ion Fe II, molecular bands of MnO oxides. Moreover, the lines of alkali metals are highly intense. There are no bands of neutral magnesium or its ion in the survey spectrum, however, in the experiment, magnesium lines were recorded in the ultraviolet region using an AvaSpec-ULS4096-CL-2-EVO spectrometer. The dynamics of the sample surface temperature, determined from the continuum in the Wien region of the spectrum, is shown in (Fig. 18). The electron temperature, calculated from the relative intensity of a number of iron lines (the method is described below), was ~5500 K. Due to the absence of TiO emission bands, despite the presence of titanium in the LMS-1D composition in the form of ilmenite, it was impossible to determine the rotational temperature of the gas using the method used for the ilmenite concentrate sample.
Based on a number of lines of iron Fe I (Table 6), the temperature was calculated based on formula (1), reduced to the form
where all parameters, except temperature, refer to one line, and the value K is the same for all lines of one source (in our case, neutral iron Fe I). Each line corresponds to a point in coordinates \(\ln \frac{{I\lambda }}{{gA}}\) and E. These points must lie on a straight line, the angle of inclination of which depends only on temperature. Specific values for each frame were calculated using the least-squares method.
Figure 19 shows microphotographs of a sample of the lunar dust simulant LMS-1D with the addition of metallic magnesium powder after exposure to a microwave discharge. Samples in Fig. 19 were taken from the inner walls of the quartz tube. Among the deposited particles there are many very fine dust and particles that do not differ in shape from the original ones. Spherical particles are also found, but less frequently than in the ilmenite concentrate sample. Moreover, quite large (~100 μm) spherical particles are also detected. On the element distribution maps (Fig. 20), it can be noted that, as in the original sample, the main constituent elements are distributed almost evenly, with the exception of magnesium, some of which was added in the form of metal powder. Local measurements of the elemental composition in different areas of the sample and for particles of different shapes clearly indicate an increase (up to two times) in the proportion of magnesium compared to the composition of the initial sample of the lunar dust simulant LMS-1D with the addition of metallic magnesium powder (Table 7), which, apparently, is associated with the spraying and subsequent deposition on the surface of other particles of metallic magnesium added in the form of powder to the simulant. Most likely, magnesium is present in an oxidized state. You can also note the formation of agglomerates of small particles or settling of small particles on large particles, in some cases to the point of completely covering the surface of large particles.
Microphotograph, with a superimposed multilayer map of the distribution of elements, and maps of the distribution of individual elements in a sample of lunar dust simulant LMS-1D with the addition of 10% metallic magnesium after exposure to a microwave discharge. Color figure is available in the electronic version of the article.
Substance of the Tsarev Meteorite
The working gas in the experiment was atmospheric-pressure air. As part of an additional experiment, titanium plates were placed in a quartz tube (Fig. 8). Their long side was located perpendicular to the electric field strength vector of linearly polarized microwave radiation. The power of microwave radiation increased from pulse to pulse from 200 to 400 kW, and the pulse duration from 4 to 6 ms. The sample was exposed to 17 microwave pulses with a total energy of 39.2 kJ. The discharge in a powder sample of the Tsarev meteorite substance was initiated with a delay of 1–2 ms from the beginning of the front of the microwave pulse. Photographs of the sample before and after exposure to the discharge are shown in Fig. 8.
The development of a discharge in the material of the Tsarev meteorite (Fig. 21) has the same stages as the two samples given above: radial expansion of the plasma formation; the stage with the highest radiation intensity and temperature of plasma–dust formation during a microwave pulse; the stage of cooling and expansion after the end of the microwave pulse. The lower intensity and duration of glow in the frames of Fig. 21, compared to Fig. 9 and Fig. 16 may be associated with the use of significantly shorter exposure times.
Footage of the development of a microwave discharge in a sample of material from the Tsarev meteorite. The frame start time (milliseconds) from the moment of breakdown is indicated on each frame in the upper left corner. Frame exposure time 0.6 μs. Microwave power 400 kW, microwave pulse duration 6 ms.
In the survey radiation spectrum (Fig. 22), for the direction of receiving radiation from bottom to top through the entire layer of the powder sample, there are many lines of neutral iron Fe I, lines of neutral titanium Ti I, calcium Ca I, lithium Li I, potassium K I, rubidium Rb I, sodium Na I, calcium ion Ca II, and molecular bands of oxides MgO and CaO. The work (Akhmadullina et al., 2023) also detected lines of the iron ion Fe II in the ultraviolet region. The spectra we obtained in the experiment have similar features with the spectra of meteors (Borovička, 1994; Vojáček et al., 2015), with the spectra of an expanding gas cloud after an impact event (Schultz and Eberhardy, 2015) and with spectra when simulating an impact event using laser ablation (Ferus et al., 2018, 2019; Hawkes et al., 2008; Křivková et al., 2021). The similarity lies in the presence of a large number of iron and calcium lines (Tables 6, 8). Among the most significant differences, it is worth highlighting: the presence of an intense thermal continuum, the absence of lines of doubly ionized iron and other metals. A detailed comparison of the spectra deserves a separate study.
The sample surface temperature values are shown in (Fig. 23). The electron temperature, calculated from the relative intensity of pairs of calcium Ca I lines (lines 422.673 and 430.253 nm), which have a significant difference in the energy of the upper level and are present in several recording time windows during one discharge, was ~7900 K. The dynamics of changes in electron temperature are given in Table 9 for calculations using the above Ca I lines (TCa) and a number of Fe I lines (TFe), (the method is given above). The calculation was carried out for those moments in time indicated in Table 9 that were both within the duration (≤4 ms) of the microwave pulse, and after its end (>4 ms). The spectrum exposure time is 0.5 ms, and the time given in Table 9 corresponds to the beginning of the spectrum accumulation time interval.
An SEM analysis of samples from the Tsarev meteorite after exposure to a microwave discharge is shown in Fig. 24. The sample taken from the inner walls of the quartz tube contains mainly spherical particles. It is worth noting the presence of spherical particles, the sizes of which (~100 μm) are larger than the sizes of the particles of the original substance. Which indicates the probable merging of microdroplets of the melt formed under the influence of a microwave discharge. The sample also contains porous particles and particles of complex shape. On the surface of large particles, submicron particles and small spherical particles adhering to them are observed. The elemental composition of the spherical particles is close to each other (Fig. 25), but local increases in the concentrations of nickel, aluminum, sulfur, and sodium within individual particles can be noted. The elemental composition of a separate sample of the material from the Tsarev meteorite after exposure to a microwave discharge is presented in Table 10 along with the elemental composition of the original sample for ease of comparison. The XRF we carried out in (Akhmadullina et al., 2023) did not show the appearance of new phases or changes in the ratio of the main phases (olivine main phase, orthopyroxene secondary) after exposure of the sample to a microwave discharge.
CONCLUSIONS
In experiments on microwave discharge in powder samples, plasma–dust clouds were obtained from substances relevant for the cosmic processes of the formation of a multiphase plasma–dust medium. The dynamics of the development of plasma–dust clouds had characteristic stages: discharge initiation; radial expansion of the plasma formation above the surface of the samples; maintaining the maximum temperature of the plasma and gas during the duration of the microwave pulse with the ongoing evaporation of the substance and the emission of sample particles into the volume; cooling and scattering after completion of the microwave pulse. In the emission spectra of plasma–dust clouds, lines of neutral atoms, lines of ions and molecular bands of oxides of substances included in the original samples were recorded. The measured electron temperatures reached 7000 K, gas temperatures 5500 K, and powder sample surface temperatures 2000 K.
The occurrence of a low-threshold microwave discharge in a powder sample of ilmenite concentrate and material from the Tsarev meteorite without adding metal powders to them is interesting in itself. This is a consequence of the sufficient intrinsic content of conducting phases in these samples for the occurrence of a discharge of this type. This fact may be necessary to take into account, for example, in applied problems of sintering lunar regolith by irradiation with microwave radiation (Taylor and Meek, 2005; Meurisse et al., 2017). When a certain threshold is exceeded (10–20 kW/cm2) based on the wave intensity and sufficient conductive phase content, a discharge can occur. Moreover, vacuum conditions should not be an obstacle for the discharge to occur, since it will develop in the cloud of its own vapors of the heated regolith. In the future, it would be interesting to conduct such an experiment under high vacuum conditions.
The result of the impact of the microwave discharge on all samples was a change in the morphology of the particles (spheroidization of the particles occurred) and, to some extent, in the sizes of the particles (individual, larger spherical particles appeared than were in the original samples). In the case of the LMS-1D lunar dust simulant sample, fewer spherical particles were observed in the samples than for the other two samples. The appearance of large submicron-sized particles on the surface was observed. At the same time, no obvious changes in the elemental composition determined by XRD for all samples were detected.
The experimental approach used in the work can be used to study the prebiological synthesis of organic substances under conditions of plasma–dust clouds with the important role of dust particles as crystallization centers and/or as a catalyst (Parmon, 1999; Peters et al., 2023; Saladino et al., 2013, 2018; Snytnikov, 2010; Snytnikov et al., 2002). To do this, it is necessary to use either minerals characteristic of space objects or the substance of space objects directly as powder samples. The working gas can be a mixture of gases assumed for the location of the process (for example, a model of the Earth’s primary atmosphere).
Plasma–dust methods turn out to be effective for studying the possibility of processes of synthesis of organic compounds in the conditions of the early Earth, in particular, during the passage of meteoric bodies in the atmosphere. During such a passage, a meteoroid trail is formed containing various phases of substances, including a dust component. Organic matter is quite easily synthesized on solid catalysts. To do this, they must contain, for example, iron, nickel and silicon, which are present in meteoric matter. Also, as a result of the development of waves and instabilities in the dusty plasma of meteoroid tails, dust particles can concentrate in local areas, which increases the likelihood of chemical reactions occurring (Morozova and Popel, 2020, 2021a, 2021b, 2022a, 2022b, 2023). The energies for excitation of vibrational degrees of freedom at temperatures reached in meteor trails make it possible for the development of free radical chemical processes and the occurrence of a wider range of chemical reactions (Gurel and Gurel, 1983). Also in protoplanetary disks, one can expect the development of processes of synthesis of organic compounds in areas of dust concentration, especially in the case of its charging and the presence of electrons and ions in the surrounding plasma, especially during outbursts of protostars (Morozova and Kuznetsov, 2023; Morozova et al., 2022c; Snytnikov al., 2002).
REFERENCES
Akhmadullina, N.S., Skvortsova, N.N., Obraztsova, E.A., Stepakhin, V.D., Konchekov, E.M., Letunov, A.A., Konovalov, A.A., Kargin, Yu.F., and Shishilov, O.N., Plasma-chemical processes under high-power gyrotron’s discharge in the mixtures of metal and dielectric powders, Chem. Phys., 2019, vol. 516, pp. 63–70. https://doi.org/10.1016/j.chemphys.2018.08.023
Akhmadullina, N.S., Borzosekov, V.D., Skvortsova, N.N., Stepakhin, V.D., Gusein-Zade, N.G., Malakhov, D.V., Knyazev, A.V., Gayanova, T.E., Kozak, A.K., Soko-lov, A.S., Sarksyan, K.A., Ishchenko, A.V., Weinstein, I.A., Grokhovsky, V.I., and Shishilov, O.N., Interaction of the substance of the Tsarev meteorite with radiation from a powerful gyrotron: Dusty plasma cloud formation and phase transformations, Fusion Sci. Technol., 2023. https://doi.org/10.1080/15361055.2023.2250669
Barsukova, L.D., Kharitonova, V.Ya., and Bannykh, L.N., Chemical composition of the Tsarev meteorite, Meteoritika, 1982, no. 41, pp. 41–43.
Basu, A., Nanophase Fe in lunar soils, J. Earth Syst. Sci., 2005, vol. 114, pp. 375–380. https://doi.org/10.1007/BF02702956
Batanov, G.M., Berezhetskaya, N.K., Kossyi, I.A., Magunov, A.N., and Silakov, V.P., Interaction of high-power microwave beams with metal-dielectric media, Eur. Phys. J. Appl. Phys., 2004, vol. 26, pp. 11–16. https://doi.org/10.1051/epjap:2004016
Batanov, G.M., Berezhetskaya, N.K., Borzosekov, V.D., Iskhakova, L.D., Kolik, L.V., Konchekov, E.M., Letunov, A.A., Malakhov, D.V., Milovich, F.O., Obraztsova, E.A., Obraztsova, E.D., Petrov, A.E., Sarksyan, K.A., Skvortsova, N.N., Stepakhin, V.D., and Kharchev, N.K., Application of microwave discharge for the synthesis of TiB2 and BN nano- and microcrystals in a mixture of Ti–B powders in a nitrogen atmosphere, Plasma Phys. Rep., 2013, vol. 39, pp. 843–848. https://doi.org/10.1134/S1063780X13100024
Batanov, G.M. and Kossyi, I.A., Pulsed microwave discharges in powder mixtures: Status, problems, and prospects, Plasma Phys. Rep., 2015, vol. 41, pp. 847–857. https://doi.org/10.1134/S1063780X15090020
Batanov, G.M., Borzosekov, V.D., Voronova, E.V., Kachmar, V.V., Kolik, L.V., Konchekov, E.M., Letunov, A.A., Malakhov, D.V., Petrov, A.E., Sarksyan, K.A., Skvortsova, N.N., Stepakhin, V.D., and Kharchev, N.K., Microwave discharge in gas above regolith surface, Plasma Phys. Rep., 2022, vol. 48, pp. 408–414. https://doi.org/10.1134/S1063780X22040031
Best, S.R. and Rose, M.F., A plasma drag hypervelocity particle accelerator (HYPER), Int. J. Impact Eng., 1999, vol. 23, no. 1, pp. 67–76. https://doi.org/10.1016/S0734-743X(99)00063-9
Bezaeva, N.S., Badjukov, D.D., Rochette, P., Gattacceca, J., Trukhin, V.I., Kozlov, E.A., and Uehara, M., Experimental shock metamorphism of the L4 ordinary chondrite Saratov induced by spherical shock waves up to 400 GPa, Meteorit. Planet. Sci., 2010, vol. 45, no. 6, pp. 1007–1020. https://doi.org/10.1111/j.1945-5100.2010.01069.x
Blanchard, M.B. and Cunningham, G.G., Artificial meteor ablation studies: Olivine, J. Geophys. Res., 1974, vol. 79, no. 26, pp. 3973–3980. https://doi.org/10.1029/JB079i026p03973
Bones, D.L., Gomez, MartinJ.C., Empson, C.J., Carrillo, SanchezJ.D., James, A.D., Conroy, T.P., and Plane, J.M.C., A novel instrument to measure differential ablation of meteorite samples and proxies: The Meteoric Ablation Simulator (MASI), Rev. Sci. Instrum., 2016, vol. 87, no. 9, p. 094504. https://doi.org/10.1063/1.4962751
Borovička, J., Two components in meteor spectra, Planet. Space Sci., 1994, vol. 42, no. 2, pp. 145–150. https://doi.org/10.1016/0032-0633(94)90025-6
Bronshten, V.A., Fizika meteornykh yavlenii (Physics of Meteor Phenomena), Moscow: Nauka, 1981.
Bykov, Yu.V., Rybakov, K.I., and Semenov, V.E., High-temperature microwave processing of materials, J. Phys. D: Appl. Phys., 2001, vol. 34, p. R55. https://doi.org/10.1088/0022-3727/34/13/201
Chhabildas, L.C., Kmetyk, L.N., Reinhart, W.D., and Hall, C.A., Enhanced hypervelocity launcher—capabilities to 16 km/s, Int. J. Impact Eng., 1995, vol. 17, nos. 1–3, pp. 183–194. https://doi.org/10.1016/0734-743X(95)99845-I
Egorov, S.V., Eremeev, A.G., Plotnikov, I.V., Rybakov, K.I., Kholoptsev, V.V., and Bykov, Yu.V., Absorption of microwaves in metal-ceramic powder materials, Radiophys. Quantum Electron., 2010, vol. 53, nos. 5–6, pp. 354–362. https://doi.org/10.1007/s11141-010-9234-8
Ferus, M., Koukal, J., Lenža, L., Srba, J., Kubelik, P., Laitl, V., Zanozina, E.M., Pavel, V., Kaiserová, T., Knížek, A., Rimmer, P., Chatzitheodoridis, E., and Civiš, S., Calibration-free quantitative elemental analysis of meteor plasma using reference laser-induced breakdown spectroscopy of meteorite samples, Astron. Astrophys., 2018, vol. 610, p. A73. https://doi.org/10.1051/0004-6361/201629950
Ferus, M., Kubelik, P., Petera, L., Lenža, L., Koukal, J., Křivková, A., Laitl, V., Knížek, A., Saeidfirozeh, H., Pastorek, A., Kalvoda, T., Juha, L., Dudžák, R., Civiš, S., Chatzitheodoridis, E., and Krůs, M., Main spectral features of meteors studied using a terawatt-class high-power laser, Astron. Astrophys., 2019, vol. 630, p. A127. https://doi.org/10.1051/0004-6361/201935816
Ferus, M., Rimmer, P., Cassone, G., Knížek, A., Civiš, S., Šponer, J.E., Ivanek, O., Šponer, J., Saeidfirozeh, H., Kubelik, P., Dudžák, R., Petera, L., Juha, L., Pastorek, A., Křivková, A., and Krůs, M., One-pot hydrogen cyanide-based prebiotic synthesis of canonical nucleobases and glycine initiated by high-velocity impacts on early Earth, Astrobiology, 2020, vol. 20, no. 12, pp. 1476–1488. https://doi.org/10.1089/ast.2020.2231
Florenskii, K.P., Bazilevskii, A.T., and Nikolaeva, O.V., Lunnyi grunt: svoistva i analogi. Model’ 1974 goda. Monografiya (Lunar Soil: Properties and Analogues. Model of 1974. Monograph), Moscow: Vseross. Inst. Nauchn. Tekh. Inf. RAN, 1975.
Friichtenicht, J.F., Two-million-volt electrostatic accelerator for hypervelocity research, Rev. Sci. Instrum., 1962, vol. 33, no. 2, pp. 209–212. https://doi.org/10.1063/1.1746548
Fulvio, D., Fuks Maron, L., Cires Perez, Y., Tahir, and Del Rosso, T., Micrometeorite bombardment simulated by ns-pulsed laser ablation: Morphological characterization of the impact craters, Icarus, 2021, vol. 366, p. 114532. https://doi.org/10.1016/j.icarus.2021.114532
Gayanova, T.E., Voronova, E.V., Kuznetsov, S.V., Obraztsova, E.A., Skvortsova, N.N., Sokolov, A.S., Nugaev, I.R., and Stepakhin, V.D., Synthesis of microstructures of hexagonal boron nitride in gyrotron discharge in metal-dielectric powder mixtures, High Energy Chem., 2023, vol. 57, no. 1 Suppl., pp. 53–56. https://doi.org/10.1134/S0018143923070111
Gerasimov, M.V. and Safonova, E.N., Shock processes role in abiotic synthesis of organics matter at the early stages of the Earth formation, in Problemy zarozhdeniya i evolyutsii biosfery (Problems of Biosphere Origin and Evolution), Galimov, E.M., Ed., Moscow: URSS, 2008, pp. 145–153.
Gerasimov, M.V., Ivanov, B.A., Yakovlev, O.I., and Dikov, Yu.P., Physics and chemistry of impacts, Earth, Moon and Planets, 1998, vol. 80, pp. 209–259. https://doi.org/10.1023/A:1006322032494
Goetz, C., Gunell, H., Volwerk, M., Beth, A., Eriksson, A., Galand, M., Henri, P., Nilsson, H., Simon, WedlundC., Alho, M., Andersson, L., Andre, N., De Keyser, J., Deca, J., Ge, Y., Glassmeier, K.-H., Hajra, R., Karlsson, T., Kasahara, S., Kolmasova, I., LLera, K., Madanian, H., Mann, I., Mazelle, C., Odelstad, E., Plaschke, F., Rubin, M., Sanchez-Cano, B., Snodgrass, C., and Vigren, E., Cometary plasma science, Exp. Astron., 2022, vol. 54, pp. 1129–1167. https://doi.org/10.1007/s10686-021-09783-z
Gómez Martín, J.C., Bones, D.L., Carrillo-Sánchez, J.D., James, A.D., Trigo-Rodríguez, J.M., Fegley, B., Jr., and Plane, J.M.C., Novel experimental simulations of the atmospheric injection of meteoric metals, Astrophys. J., 2017, vol. 836, p. 212. https://doi.org/10.3847/1538-4357/aa5c8f
Grokhovsky, V.I., Muftakhetdinova, R.F., Yakovlev, G.A., Brusnitsyna, E.V., and Petrova, E.V., Post-impact metamorphism of the Chelyabinsk meteorite in shock experiment, Planet. Space Sci., 2020, vol. 192, p. 105050. https://doi.org/10.1016/j.pss.2020.105050
Grunt iz materikovogo raiona Luny (Soil from the Contniental Region of the Moon), Barsukov, V.L. and Surkov, Yu.A., Eds., Moscow: Nauka, 1979.
Gurel, D. and Gurel, O., Oscillations in Chemical Reactions, Berlin: Springer-Verlag, 1983.
Hawkes, R.L., Milley, E.P., Ehrman, J.M., Woods, R.M., Hoyland, J.D., Pettipas, C.L., and Tokaryk, D.W., What can we learn about atmospheric meteor ablation and light production from laser ablation?, Earth, Moon and Planet, 2008, vol. 102, pp. 331–336. https://doi.org/10.1007/s11038-007-9186-y
Heiken, G., Vaniman, D., and French, B., Lunar Sourcebook, Cambridge: Cambridge Univ. Press, 1991, pp. 286–356.
Helber, B., Dias, B., Bariselli, F., Zavalan, L.F., Pittarello, L., Goderis, S., Soens, B., McKibbin, S.J., Claeys, P., and Magin, T.E., Analysis of meteoroid ablation based on plasma wind-tunnel experiments, surface characterization, and numerical simulations, Astrophys. J., 2019, vol. 876, no. 2, p. 120. https://doi.org/10.3847/1538-4357/ab16f0
Hermann, J., Perrone, A., and Dutouquet, C., Analyses of the TiO-γ system for temperature measurements in a laser-induced plasma, J. Phys. B, 2001, vol. 34, pp. 153–164. https://doi.org/10.1088/0953-4075/34/2/303
Hudepohl, A., Rott, M., and Igenbergs, E., Coaxial plasma accelerator with compressor coil and radial gas injection, IEEE Trans. Magn., 1989, vol. 25, pp. 232–237. https://doi.org/10.1109/20.22540
Igenbergs, E., Aigner, S., Hüdepohl, A., Iglseder, H., Kuczera, H., Rott, M., and Weishaupt, U., Launcher technology, in-flight velocity measurement and impact diagnostics at the TUM/LRT, Int. J. Impact Eng., 1987, vol. 5, nos. 1–4, pp. 371–385. https://doi.org/10.1016/0734-743X(87)90054-6
Jenniskens, P., Meteors as a delivery vehicle for organic matter to the early Earth, Meteoroids 2001 Conf., ESA Special Publication, 2001, vol. 495, pp. 247–254.
Jenniskens, P., Schaller, E.L., Laux, C.O., Wilson, M.A., Schmidt, G., and Rairden, R.L., Meteors do not break exogenous organic molecules into high yields of diatomics, Astrobiology, 2004, vol. 4, no. 1, pp. 67–79. https://doi.org/10.1089/153110704773600249
Johnstone, A.D., Cometary Plasma Processes. Geophysical Monograph Series, American Geophysical Union, 2013.
Kachmar, V.V., Moshkina, K.G., Borzosekov, V.D., Sorokin, A.A., and Skvortsova, N.N., Non-Gaussian distribution of regolith particles deposited on tantalum and molybdenum surfaces under gyrotron pulsed radiation, J. Phys.: Conf. Ser., 2021, vol. 2036, p. 012030. https://doi.org/10.1088/1742-6596/2036/1/012030
Károly, Z. and Szépvölgyi, J., Plasma spheroidization of ceramic particles, Chem. Eng. Process.: Process Intensif., 2005, vol. 44, no. 2, pp. 221–224.
Křivková, A., Petera, L., Laitl, V., Kubelik, P., Chatzitheodoridis, E., Lenža, L., Kouka, J., Knížek, A., Dudžák, R., Páclík, D., Civiš, S., Krůs, M., and Ferus, M., Application of a dielectric breakdown induced by high-power lasers for a laboratory simulation of meteor plasma, Exp. Astron., 2021, vol. 51, pp. 425–451. https://doi.org/10.1007/s10686-020-09688-3
Kuznetsov, I.A., Zakharov, A.V., Dolnikov, G.G., Lyash, A.N., Afonin, V.V., Popel, S.I., Shashkova I.A., and Borisov, N.D., Lunar dust: Properties and investigation techniques, Sol. Syst. Res., 2016, vol. 51, pp. 611–622.
Kuznetsov, I.A., Zakharov, A.V., Zelenyi, L.M., Popel, S.I., Morozova, T.I., Shashkova, I.A., Dolnikov, G.G., Lyash, A.N., Dubov, A.E., Viktorov, M.E., Topchieva, A.P., Klumov, B.A., Usachev, A.D., Lisin, E.A., Vasiliev, M.M., Petrov, O.F., and Poroikov, A.Yu., Dust particles in space: Opportunities for experimental research, Astron. Rep., 2023, vol. 67, pp. 35–60. https://doi.org/10.1134/S1063772923010110
Lexow, B., Wickert, M., Thoma, K., Schafer, F., Poelchau, M.H., and Kenkmann, T., The extra-large light-gas gun of the Fraunhofer EMI: Applications for impact cratering research, Meteorit. Planet. Sci., 2013, vol. 48, pp. 3–7. https://doi.org/10.1111/j.1945-5100.2012.01427.x
Litvak, A.G., Denisov, G.G., and Glyavin, M.Y., Russian gyrotrons: Achievements and trends, IEEE J. Microwaves, 2021, vol. 1, no. 1, pp. 260–268. https://doi.org/10.1109/JMW.2020.3030917
Loehle, S., Zander, F., Hermann, T., Eberhart, M., Meindl, A., Oefele, R., Vaubaillon, J., Colas, F., Vernazza, P., Drouard, A., and Gattacceca, J., Experimental simulation of meteorite ablation during earth entry using a plasma wind tunnel, Astrophys. J., 2017, vol. 837, p. 112. https://doi.org/10.3847/1538-4357/aa5cb5
Lochte-Holtgreven, W., Plasma Diagnostics, Amsterdam: North-Holland, 1968.
Long-Fox, J.M. and Britt, D.T., Characterization of planetary regolith simulants for the research and development of space resource technologies, Front. Space Technol., 2023, vol. 4, p. 1255535. https://doi.org/10.3389/frspt.2023.1255535
Lunnyi grunt iz Morya Izobiliya (Lunar Soil from Mare Fecunditatis), Vinogradov A.P., Ed., Moscow: Nauka, 1974.
Lunnyi grunt iz Morya Krizisov (Lunar Soil from Mare Crisium), Barsukov V.L., Ed., Moscow: Nauka, 1980.
Madison, A., Landsman, Z., Long-Fox, J., Metke, A., Krol, K., Easter, P., Sipe, C., Weber, L., and Britt, D., Lunar dust simulants and their applications, Conf. Earth and Space, 2022, pp. 86–94. https://doi.org/10.1061/9780784484470.008
Maksimova, A.A., Oshtrakh, M.I., Klencsar, Z., Petrova, E.V., Grokhovsky, V.I., Kuzmann, E., Homonnay, Z., and Semionkin, V.A., A comparative study of troilite in bulk ordinary chondrites Farmington L5, Tsarev L5 and Chelyabinsk LL5 using Mössbauer spectroscopy with a high velocity resolution, J. Mol. Struct., 2014, vol. 1073, pp. 196–201. https://doi.org/10.1016/j.molstruc.2014.05.049
Maksimova, A.A., Oshtrakh, M.I., Petrova, E.V., Grokhovsky, V.I., and Semionkin, V.A., Comparison 263 of iron-bearing minerals in ordinary chondrites from H, L and LL groups using Mössbauer spectroscopy with a high velocity resolution, Spectrochim. Acta. A: Mol. Biomol. Spectrosc., 2017, vol. 172, pp. 65–76. https://doi.org/10.1016/j.saa.2016.04.032
Maksimova, A.A., Kamalov, R.V., Chukin, A.V., Felner, I., and Oshtrakh, M.I., An analysis of orthopyroxene from Tsarev L5 meteorite using X-ray diffraction, magnetization measurement and Mössbauer spectroscopy, J. Mol. Struct., 2018, vol. 1174, pp. 6–11. https://doi.org/10.1016/j.molstruc.2018.06.040
Managadze, G.G., The synthesis of organic molecules in a laser plasma similar to the plasma that emerges in hypervelocity collisions of matter at the early evolutionary stage of the Earth and in interstellar clouds, J. Exp. Theor. Phys., 2003, vol. 97, pp. 49–60. https://doi.org/10.1134/1.1600796
Martins, Z., Price, M., Goldman, N., Sephton, M.A., and Burchell, M.J., Shock synthesis of amino acids from impacting cometary and icy planet surface analogues, Nat. Geosci., 2013, vol. 6, pp. 1045–1049. https://doi.org/10.1038/ngeo1930
Meurisse, A., Beltzung, J.C., Kolbe, M., Cowley, A., and Sperl, M., Influence of mineral composition on sintering lunar regolith, J. Aerosp. Eng., 2017, vol. 30, no. 4, p. 113695951. https://doi.org/10.1061/(ASCE)AS.1943-5525.0000721
Migdisova, L.F., Zaslavskaya, N.I., Ivanov, A.V., Shcherbovskii, E.Ya., and Levskii, L.K., Features of the composition and structure of the Tsarev meteorite, Meteoritika, 1982, no. 41, pp. 13–30.
Mocker, A., Bugiel, S., Auer, S., Baust, G., Colette, A., Drake, K., Fiege, K., Grün, E., Heckmann, F., Helfert, S., Hillier, J., Kempf, S., Matt, G., Mellert, T., Munsat, T., Otto, K., Postberg, F., Roser, H.-P., Shu, A., Sternovsky, Z., and Srama, R., A 2 MV Van de Graaff accelerator as a tool for planetary and impact physics research, Rev. Sci. Instrum., 2011, vol. 82, no. 9, p. 095111. https://doi.org/10.1063/1.3637461
Moroz, L.V., Fisenko, A.V., Semjonova, L.F., Pieters, C.M., and Korotaeva, N.N., Optical effects of regolith processes on S-asteroids as simulated by laser shots on ordinary chondrite and other mafic materials, Icarus, 1996, vol. 122, no. 2, pp. 366–382. https://doi.org/10.1006/icar.1996.0130
Morozova, T.I. and Kuznetsov, I.A., Photoelectric charging of dust in protoplanetary disks, Moscow Univ. Phys. Bull., 2023, vol. 78, no. 3, pp. 388–391.
Morozova, T.I. and Popel, S.I., On the plasma–dust processes accompanying meteor showers, Plasma Phys. Rep., 2020, vol. 46, pp. 1075–1088. https://doi.org/10.1134/S1063780X20100074
Morozova, T.I. and Popel, S.I., Electrophonic noises from meteors and dust acoustic modulational perturbations, J. Phys. Conf. Ser., 2021a, vol. 1787, p. 012052. https://doi.org/10.1088/1742-6596/1787/1/012052
Morozova, T.I. and Popel, S.I., Modulational interaction in a dusty plasma of meteoroid wakes, Geomagn. Aeron., 2021b, vol. 61, pp. 888–895. https://doi.org/10.1134/S0016793221060116
Morozova, T.I. and Popel, S.I., Lower hybrid waves upon interaction of meteor wakes with the Earth’s ionosphere, Plasma Phys. Rep., 2022a, vol. 48, pp. 774–777. https://doi.org/10.1134/S1063780X22600384
Morozova, T.I. and Popel, S.I., Modulational interaction of Langmuir waves and generation of magnetic field in meteoroid trails, Plasma Phys. Rep., 2022b, vol. 48, pp. 1075–1079. https://doi.org/10.1134/S1063780X22600414
Morozova, T.I. and Popel, S.I., Some aspects of modulational interaction in Earth’s dusty ionosphere including dusty plasmas of meteor tails, Plasma Phys. Rep., 2023, vol. 49, pp. 65–69. https://doi.org/10.1134/S1063780X22601389
Morozova, T.I., Garasev, M.A., and Kuznetsov, I.A., On the possibility of dust grain destruction by the coloumb explosion in protoplanetary disks, Radiophys. Quantum Electron., 2022c, vol. 65, pp. 1–8. https://doi.org/10.1007/s11141-022-10188-x
NASA Lunar Sample and Photo Catalog. https://curator.jsc.nasa.gov/lunar/samplecatalog/. Accessed November 13, 2023.
Oshtrakh, M.I., Petrova, E.V., Grokhovsky, V.I., and Semionkin, V.A., A study of ordinary chondrites by Mössbauer spectroscopy with high-velocity resolution, Meteorit. Planet. Sci., 2008, vol. 43, no. 5, pp. 941–958. https://doi.org/10.1111/j.1945-5100.2008.tb01091.x
Oshtrakh, M.I., Petrova, E.V., and Grokhovsky, V.I., and Semionkin, V.A., Variations in quadrupole splitting of the 57Fe in the M1 and M2 sites of meteoritic olivines with different origin, Hyperfine Interact., 2013a, vol. 222, pp. 61–66. https://doi.org/10.1007/s10751-012-0694-4
Oshtrakh, M.I., Grokhovsky, V.I., Petrova, E.V., Larionov, M.Yu., Goryunov, M.V., and Semionkin, V.A., Mossbauer spectroscopy with a high velocity resolution applied for the study of meteoritic iron-bearing minerals, J. Mol. Struct., 2013b, vol. 1044, pp. 268–278. https://doi.org/10.1016/j.molstruc.2012.09.059
Park, J., Liu, Y., Kihm, K.D., Hill, E., and Taylor, L.A., Submicron particle size distribution of Apollo 11 lunar dust, in Earth and Space 2006: Engineering, Construction, and Operations in Challenging Environment, 2006, pp. 1–6. https://doi.org/10.1061/40830(188)2
Parmon, V.N., Abiogenic catalysis in nature, Colloids Surf. A: Physicochem. Eng. Aspects, 1999, vol. 151, nos. 1–2, pp. 351–365. https://doi.org/10.1061/40830(188)27
Peters, S., Semenov, D.A., Hochleitner, R., and Trapp, O., Synthesis of prebiotic organics from CO2 by catalysis with meteoritic and volcanic particles, Sci. Rep., 2023, vol. 13, p. 6843. https://doi.org/10.1038/s41598-023-33741-8
Popel, S.I., Zelenyi, L.M., Golub’, A.P., and Dubinskii, A.Yu., Lunar dust and dusty plasmas: Recent developments, advances, and unsolved problems, Planet. Space Sci., 2018, vol. 156, pp. 71–84. https://doi.org/10.1016/j.pss.2018.02.010
Popel, S.I., Golub’, A.P., Zakharov, A.V., and Zelenyi, L.M., Formation of microspherules of lunar regolith in plasma-dust processes initiated by meteoroid impacts, Plasma Phys. Rep., 2020, vol. 46, pp. 265–272. https://doi.org/10.1134/S1063780X20030101
Prince, B.S., Magnuson, M.P., Chaves, L.C., Thompson, M.S., and Loeffler, M.J., Space weathering of FeS induced via pulsed laser irradiation, J. Geophys. Res.: Planets, 2020, vol. 125, no. 5, p. e2019JE006242. https://doi.org/10.1029/2019JE006242
Raizer, Yu.P., Fizika gazovogo razryada (Physics of Gas Discharge), Dolgoprudnyi: Izd. dom Intellekt, 2009.
Rybakov, K.I. and Buyanova, M.N., Microwave resonant sintering of powder metals, Scripta Materialia, 2018, vol. 149, pp. 108–111. https://doi.org/10.1016/j.scriptamat.2018.02.014
Rybakov, K.I., Semenov, V.E., Egorov, S.V., Eremeev, A.G., Plotnikov, I.V., and Bykov, Yu.V., Microwave heating of conductive powder materials, J. Appl. Phys., 2006, vol. 99, no. 2, p. 023506. https://doi.org/10.1063/1.2159078
Rybakov, K.I., Olevsky, E.A., and Krikun, E.V., Microwave sintering: Fundamentals and modeling, J. Am. Ceram. Soc., 2013, vol. 96, no. 4, pp. 1003–1020. https://doi.org/10.1111/jace.12278
Saladino, R., Botta, G., Delfino, M., and Di Mauro, E., Meteorites as catalysts for prebiotic chemistry, Chem.-Eur. J., 2013, vol. 19, no. 50, pp. 16916–16922. https://doi.org/10.1002/chem.201303690
Saladino, R., Botta, L., and Di Mauro, E., The prevailing catalytic role of meteorites in formamide prebiotic processes, Life, 2018, vol. 8, no. 1, p. 6. https://doi.org/10.3390/life8010006
Samokhin, A.V., Fadeev, A.A., Alekseev, N.V., Sinaiskii, M.A., Sufiyarov, V.Sh., Borisov, E.V., Korznikov, O.V., Fedina, T.V., Vodovozova, G.S., and Baryshkov, S.V., Spheroidization of iron-based powders in the plasma flow of an electric arc plasmatron and their application in selective laser melting, Fiz. Khim. Obrab. Mater., 2019, no. 4, pp. 12–20. https://doi.org/10.30791/0015-3214-2019-4-12-20
Sasaki, S., Kurahashi, E., Yamanaka, C., and Nakamura, K., Laboratory simulation of space weathering: Changes of optical properties and TEM/ESR confirmation of nanophase metallic iron, Adv. Space Res., 2003, vol. 31, no. 12, pp. 2537–2542. https://doi.org/10.1016/S0273-1177(03)00575-1
Schultz, P.H. and Eberhardy, C.A., Spectral probing of impact-generated vapor in laboratory experiments, Icarus, 2015, vol. 248, pp. 448–462. https://doi.org/10.1016/j.icarus.2014.10.041
Semenova, L.F., Fisenko, A.V., Kashkarova, V.G., Mel’nikova, L.N., Bezrogova, E.V., Pomytkina, V.A., and Lavrukhina, A.K., Study of the chemical composition of mineral fractions of the Tsarev chondrite, Meteoritika, 1984, no. 43, pp. 114–122.
Semkin, N.D., Piyakov, A.V., Voronov, K.E., Bogoyavlenskii, N.L., and Goryunov, D.V., A linear accelerator for simulating micrometeorites, Instrum. Exp. Tech., 2007, vol. 50, pp. 275–281. https://doi.org/10.1134/S0020441207020194
Semkin, N.D., Voronov, K.E., Piyakov, A.V., and Piyakov, I.V., Simulation of micrometeorites using an electrodynamical accelerator, Instrum. Exp. Tech., 2009, vol. 52, pp. 595–601. https://doi.org/10.1134/S0020441209040228
Shu, A., Collette, A., Drake, K., Grun, E., Horányi, M., Kempf, S., Mocker, A., Munsat, T., Northway, P., Srama, R., Sternovsky, Z., and Thomas, E., 3 MV hypervelocity dust accelerator at the Colorado Center for Lunar Dust and Atmospheric Studies, Rev. Sci. Instrum., 2012, vol. 83, p. 075108. https://doi.org/10.1063/1.4732820
Silber, E.A., Boslough, M., Hocking, W.K., Gritsevich, M., and Whitaker, R.W., Physics of meteor generated shock waves in the Earth’s atmosphere—a review, Adv. Space Res., 2018, vol. 62, no. 3, pp. 489–532. https://doi.org/10.1016/j.asr.2018.05.010
Skvortsova, N.N., Malakhov, D.V., Stepakhin, V.D., Maiorov, S.A., Batanov, G.M., Borzosekov, V.D., Konchekov, E.M., Kolik, L.V., Letunov, A.A., Obraztsova, E.A., Petrov, A.E., Pozdnyakov, D.O., Sarksyan, K.A., Sorokin, A.A., Ukryukov, G.V., and Kharchev, N.K., Initiation of dusty structures in chain reactions under the action of gyrotron radiation on a mixture of metal and dielectric powders with an open boundary, JETP Lett., 2017, vol. 106, pp. 262–267. https://doi.org/10.1134/S0021364017160135
Skvortsova, N.N., Maiorov, S.A., Malakhov, D.V., Stepakhin, V.D., Obraztsova, E.A., Kenzhebekova, A.I., and Shishilov, O.N., On the dust structures and chain reactions induced over the regolith by gyrotron radiation, JETP Lett., 2019, vol. 109, no. 7, pp. 441–448. https://doi.org/10.1134/S0021364019070130
Skvortsova, N.N., Shishilov, O.N., Akhmadullina, N.S., Konchekov, E.M., Letunov, A.A., Malakhov, D.V., Obraztsova, E.A., and Stepakhin, V.D., Synthesis of micro- and nanostructured materials via oscillating reactions initiated by high-power microwave pulses, Ceram. Int., 2021a, vol. 47, pp. 3978–3987. https://doi.org/10.1016/j.ceramint.2020.09.262
Skvortsova, N.N., Stepakhin, V.D., Sorokin, A.A., Malakhov, D.V., Gusein-zade, N.G., Akhmadullina, N.S., Borzosekov, V.D., Voronova, E.V., and Shishilov, O.N., Microwave simulation experiments on regolith (lunar dust) deposition on stainless steel, Materials, 2021b, vol. 14, p. 6472. https://doi.org/10.3390/ma14216472
Skvortsova, N.N., Obraztsova, E.A., Stepakhin, V.D., Konchekov, E.M., Gayanova, T.E., Vasilieva, L.A., Lukianov, D.A., Sybachin, A.V., Skvortsov, D.A., Gusein-Zade, N.G., and Shishilov, O.N., Microdispersed Ti/B/N materials synthesized in chain reactions in processes initiated by microwaves of a high-power gyrotron: Structure and cytotoxicity, Fusion Sci. Technol., 2023a. https://doi.org/10.1080/15361055.2023.2255442
Skvortsova, N.N., Stepakhin, V.D., Borzosekov, V.D., Sorokin, A.A., Malakhov, D.V., Kachmar, V.V., Kolik, L.V., Konchekov, E.M., Gusein-zade, N.G., Akmadullina, N.S., Voronova, E.V., and Shishilov, O.N., Microwave plasma imitation experiments on deposition of lunar dust on metal plates, Plasma Phys. Rep., 2023b, vol. 49, pp. 120–128. https://doi.org/10.1134/S1063780X22601833
Slyuta, E.N., Physical and mechanical properties of the lunar soil (a review), Sol. Syst. Res., 2014, vol. 48, pp. 330–353. https://doi.org/10.1134/S0038094614050050
Slyuta, E.N., Physical and mechanical properties of stony meteorites, Sol. Syst. Res., 2017, vol. 51, pp. 64–85. https://doi.org/10.1134/S0038094617010051
Snytnikov, V.N., Dudnikova, G.I., Gleaves, J.T., Nikitin, S.A., Parmon, V.N., Stoyanovsky, V.O., Vshivkov, V.A., Yablonsky, G.S., and Zakharenko, V.S., Space chemical reactor of protoplanetary disk, Adv. Space Res., 2002, vol. 30, no. 6, pp. 1461–1467. https://doi.org/10.1016/S0273-1177(02)00507-0
Snytnikov, V.N., Astrocatalysis abiogenic synthesis and chemical evolution at pregeological stages of the Earth’s formation, Paleontol. J., 2010, vol. 44, pp. 761–777. https://doi.org/10.1134/S0031030110070063
Sokolov, A.S., Akhmadullina, N.S., Borzosekov, V.D., Voronova, E.V., Gayanova, T.É., Gusein-zade, N.G., Zakletskii, Z.A., Kozak, A.K., Malakhov, D.V., Skvortsova, N.N., Stepakhin, V.D., Obraztsova, E.A., and Shishilov, O.N., Plasma-chemical facility for synthesis of micro- and nanoparticles having controlled compositions and structures on the basis of a microwave discharge in the gyrotron radiation, Radiophys. Quantum Electron., 2023, vol. 65, pp. 840–854. https://doi.org/10.1007/s11141-023-10261-z
Sorokin, E.G., Yakovlev, O.I., Slyuta, E.N., Gerasimov, M.V., Zaitsev, M.A., Shcherbakov, V.D., Ryazantsev, K.M., and Krasheninnikov, S.P., Experimental modeling of a micrometeorite impact on the Moon, Geochem. Int., 2020a, vol. 58, pp. 113–127. https://doi.org/10.1134/S0016702920020111
Sorokin, E.M., Yakovlev, O.I., Slyuta, E.N., Gerasimov, M.V., Zaitsev, M.A., Shcherbakov, V.D., Ryazantsev, K.M., and Krasheninnikov, S.P., Experimental model of the formation of nanophase metallic iron in the lunar regolith, Dokl. Earth Sci., 2020b, vol. 492, pp. 431–433. https://doi.org/10.1134/S1028334X20060203
Stockstill-Cahill, K., Blewett, D.T., Benjamin, D., Bussey, J., Cahill, J.T.S., Clyde, B., Denevi, B.W., Hibbitts, K., Graziano, M., Greenhagen, B.T., Martin, A.C., Montalbano, T.J., Patterson, G.W., Stickle, A.M., and Wagoner, C.M., 2021 Lunar Simulant Assessment, JHU-APL LSII Report, Johns Hopkins Applied Physics Laboratory, 2021. https://lsic.jhuapl.edu/Our-Work/ Working-Groups/files/Lunar-Simulants/2021%20Lunar%20Simulant%20Assessment_final.pdf. Accessed November 13, 2023.
Stockstill-Cahill, K., Martin, A., and Wagoner, C., 2022 Lunar Simulant Assesment, JHU-APL LSII Report, Johns Hopkins Applied Physics Laboratory, 2022. https://lsic.jhuapl.edu/Our-Work/Working-Groups/ files/Lunar-Simulants/2022%20Lunar%20Simulants%-20Assessment%20Final.pdf. Accessed November 13, 2023.
Stöffler, D. and Langenhorst, F., Shock metamorphism of quartz in nature and experiment: I. Basic observation and theory, Meteorit. Planet. Sci., 1994, vol. 29, no. 2, pp. 155–181. https://doi.org/10.1111/j.1945-5100.1994.tb00670.x
Taylor, L.A., Pieters, C.M., Keller, L.P., Morris, R.V., and McKay, D.S., Lunar mare soils: Space weathering and the major effects of surface-correlated nanophase Fe, J. Geophys. Res.: Planets, 2001, vol. 106, no. E11, pp. 27985–27999. https://doi.org/10.1029/2000JE001402
Taylor, L.A. and Meek, T.T., Microwave sintering of lunar soil: Properties, theory, and practice, J. Aerosp. Eng., 2005, vol. 18, no. 3, pp. 188–196. https://doi.org/10.1061/(ASCE)0893-1321(2005)18:3(188)
Thornhill, T.F., Chhabildas, L.C., Reinhart, W.D., and Davidson, D.L., Particle launch to 19 km/s for micro-meteoroid simulation using enhanced three-stage light gas gun hypervelocity launcher techniques, Int. J. Impact Eng., 2006, vol. 33, pp. 799–811. https://doi.org/10.1016/j.ijimpeng.2006.09.015
Vasyunin, A.I., Semenov, D., Henning, Th., Wakelam, V., Herbst, E., and Sobolev, A.M., Chemistry in protoplanetary disks: A sensitivity analysis, Astrophys. J., 2008, vol. 672, pp. 629–641. https://doi.org/10.1086/523887
Vodop’yanov, A.V., Mansfeld, D.A., Samokhin, A.V., Alekseev, N.V., and Tsvetkov, Yu.V., Production of nanopowders by the evaporation-condesation method using a focused microwave radiation, Radiophys. Quantum Electron., 2017, vol. 59, pp. 698–705. https://doi.org/10.1007/s11141-017-9737-7
Vojáček, V., Borovička, J., Koten, P., Spurný, P., and Štork, R., Catalogue of representative meteor spectra, Astron. Astrophys., 2015, vol. 580, p. A67. https://doi.org/10.1051/0004-6361/201425047
Voronova, E.V., Knyazev, A.V., Letunov, A.A., Logvinenko, V.P., Skvortsova, N.N., and Stepakhin, V.D., Temperature of the surface of powders in experiments with chain plasma-chemical reactions initiated by the radiation of a gyrotron in Pd + Al2O3 mixtures, Phys. Atom. Nuclei, 2021, vol. 84, no. 10, pp. 1761–1764. https://doi.org/10.1134/S1063778821090374
Vricella, A., Delfini, A., Pacciani, A., Pastore, R., Micheli, D., Rubini, G., Marchetti, M., and Santoni, F., A new advanced railgun system for debris impact study, Procedia Struct. Integr., 2017, vol. 3, pp. 545–552. https://doi.org/10.1016/j.prostr.2017.04.044
Zaitsev, M.A., Gerasimov, M.V., Safonova, E.N., and Vasiljeva, A.S., Peculiarities in the formation of complex organic compounds in a nitrogen-methane atmosphere during hypervelocity impacts, Sol. Syst. Res., 2016, vol. 50, pp. 113–129. https://doi.org/10.1134/S0038094616020076
Zakharov, A.V., Zelenyi, L.M., Popel’, S.I., Lunar dust: Properties and potential hazards, Sol. Syst. Res., 2020, vol. 54, pp. 455–476. https://doi.org/10.1134/S0038094620060076
Funding
The authors thank the Russian Science Foundation for financial support, project no. 21-72-10164.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Borzosekov, V.D., Akhmadullina, N.S., Sokolov, A.S. et al. Obtaining Plasma–Dust Clouds from Meteoritic Matter, its Analogs and Simulants of Lunar Regolith Using Microwave Discharge. Sol Syst Res 58, 289–314 (2024). https://doi.org/10.1134/S0038094624700138
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0038094624700138