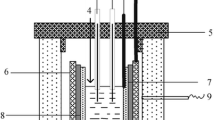
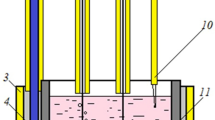
Abstract
KF–AlF3–Al2O3-based melts are promising media for the electrolytic production of aluminum in next-generation energy efficient cells. This work analyzes the dissolution of Al2O3 in the KF–AlF3 melt with a mole ratio [KF]/[AlF3] = 1.5 mol/mol at 785°C using cyclic voltammetry and the carbothermic reduction of melt samples using a LECO analyzer. The measurements are performed by a cell consisting of a carbon glass working electrode, a CO/CO2 gas reference electrode, and a graphite counter electrode. During measurements, the current response peak on voltammograms is recorded as a function of the potential scan rate, the dissolution time of the next alumina sample, and the alumina content in the melt. The current response peak is shown to linearly depend on the Al2O3 content in the melt, and the oxide dissolution rate is from 2.4 × 10–3 to 5.45 × 10–5 mol/s as a function of the oxide content in the melt. The obtained results demonstrate general possibility of operating nondestructive control of the alumina (Al2O3) content during the electrolysis of KF–AlF3–Al2O3–based melts. It includes the recording of a current response peak in current–voltage curves and the determination of the current alumina content in a melt using the obtained empirical dependence.







Similar content being viewed by others
REFERENCES
Yu. V. Borisoglebskii, G. V. Galevskii, N. M. Kulagin, and M. Ya. Mintsis, Metallurgy of Aluminum (Nauka, Novosibirsk, 1999).
L. Bracamonte, K. Nilsen, Ch. Rosenkilde, and E. Sandes, “Alumina concentration measurements in cryolite melts,” TMS: Light Metals, 600–607 (2020).
E. Skybakmoen, A. Solheim, and A. Sterten, Metall. Mater. Trans. B 27, 81–86 (1997).
J. Yang, J. N. Hryn, B. R. Davis, A. Roy, G. K. Krumdick, J. A. Pomykala Jr., “New opportunities for aluminium electrolysis with metal anodes in a low temperature electrolyte system,” TMS: Light Metals, 321–326 (2004).
A. Yu. Nikolaev, A. S. Yasinskii, A. V. Suzdaltsev, P. V. Polyakov, and Yu. P. Zaikov, “Electrolysis of aluminum in KF–AlF3–Al2O3 melts and suspensions,” Rasplavy, No. 3, 205–213 (2017).
A. S. Yasinskiy, A. V. Suzdaltsev, S. K. Padamata, P. V. Polyakov, and Yu. P. Zaikov, “Electrolysis of low–temperature suspensions: an update,” TMS: Light Metals, 626–636 (2020).
N. V. Vasyunina, I. P. Vasyunina, Yu. G. Mikhalev, and A. M. Vinogradov, “Solubility and dissolution rate of alumina in acidic cryolite–alumina melts,” Izv. Vyssh. Uchebn. Zaved., Tsvetn. Metall. 4, 24–28 (2009).
A. V. Frolov, A. O. Gusev, Yu. P. Zaikov, A. P. Khramov, N. I. Shurov, O. Yu. Tkacheva, A. P. Apisarov, and V. A. Kovrov, “Modified alumina–cryolite bath with high electrical conductivity and dissolution rate of alumina,” TMS: Light Metals, 571–576 (2007).
E. J. Frazer and J. Thonstad, Metall. Mater. Trans. B 41, 543–548 (2010).
N. E. Richards, S. Rolseth, J. Thonstad, and R. G. Haverkamp, “Electrochemical analysis of alumina dissolved in cryolite melts,” TMS: Light Metals, 391–404 (1995).
R. G. Haverkamp, S. Rolseth, J. Thonstad, and H. Gudbrandsen, “Voltammetry and electrode reactions in AlF3-rich electrolyte,” TMS: Light Metals, 481–486 (2001).
A. Yu. Nikolaev, O. B. Pavlenko, A. V. Suzdaltsev, and Yu. P. Zaikov, J. El. Chem. Soc. (2020) (in print).
R. K. Jain, H. C. Gaur, E. J. Frazer, and B. J. Welch, J. El. Analyt. Chem. 78, 1–30 (1977).
V. N. Nekrasov, O. V. Limanovskaya, A. V. Suzdaltsev, Yu. P. Zaikov, and A. P. Khramov, “Carbon anode chronopotentiometry in KF–AlF3–Al2O3 melts,” Rasplavy, No. 2, 18–29 (2011).
A. E. Dedyukhin, A. P. Apisarov, O. Yu. Tkacheva, A. A. Redkin, Yu. P. Zaikov, A. V. Frolov, and A. O. Gusev, “The electrical conductivity of the molten system [(KF–AlF3)–NaF]–Al2O3,” Rasplavy, No. 2, 23–28 (2009).
A. A. Kataev, O. Yu. Tkacheva, I. D. Zakiryanova, A. A. Apisarov, A. E. Dedyukhin, and Yu.P. Zaikov, J. Mol. Liq. 231, 149–153 (2017). https://doi.org/10.1016/j.molliq.2017.02.021
A. S. Yasinskiy, A. V. Suzdaltsev, P. V. Polyakov, S. K. Padamata, and O. V. Yushkova, Cer. Inter. B 46 (8), 11539–11548 (2020). https://doi.org/10.1016/j.ceramint.2020.01.180
V. A. Kovrov, A. P. Khramov, Yu. P. Zaikov, and N. I. Shurov, Rus. J. El. Chem. 43, 909–919 (2007).
A. V. Suzdaltsev, V. N. Nekrasov, Yu. P. Zaikov, A. P. Khramov, and O. V. Limanovskaya, “Anodic polarization on glassy carbon in low-melting potassium cryolite–alumina melts,” Rasplavy, No. 4, 41–51 (2009).
H.-M. Kan, N. Zhang, and X. Wang, J. Cent. South Univ. 19, 897–902 (2012).
L. A. Isaeva, A. B. Braslavskii, and P. V. Polyakov, Izv. Vyssh. Uchebn. Zaved., Tsvetn. Metall. 6, 35–41 (2009).
B. J. Welch and G. I. Kuschel, JOM 5, 50–54 (2007).
A. Yu. Nikolaev, A. V. Suzdaltsev, and Yu. P. Zaikov, “A new method for the synthesis of Al–Sc master alloys in oxide–fluoride and fluoride melts,” Rasplavy, No. 2, 155–165 (2020).
A. V. Suzdaltsev, A. P. Khramov, and Yu. P. Zaikov, “Carbon electrode for electrochemical studies in cryolite–alumina melts at 700–960°C,” Electrokhimiya 48 (12), 1251–1263 (2012).
ACKNOWLEDGMENTS
We are grateful to O.B. Pavlenko for the LECO analysis of samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by I. Moshkin
Rights and permissions
About this article
Cite this article
Pershin, P.S., Suzdaltsev, A.V. & Zaikov, Y.P. Dissolution of Al2O3 in KF–AlF3 . Russ. Metall. 2021, 213–218 (2021). https://doi.org/10.1134/S0036029521020191
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029521020191