Abstract
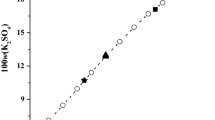
Experimental studies on the solubility, density and refractive index in the ternary system (Na2B4O7 + Mg2B6O11 + H2O) at 308.15 K were determined with the method of isothermal dissolution equilibrium. Based on the experimental results, the phase diagram and its corresponding physicochemical properties versus composition diagram in the system were plotted. In the phase diagram of the ternary system at 308.15 K, there are one eutectic point and two crystallization regions corresponding to the large area of inderite (Mg2B6O11 · 15H2O) and the relative small area of borax (Na2B4O7 · 10H2O), respectively. Neither double salt nor solid solution was found in this system. The physicochemical properties (density and refractive index) of the ternary system at 308.15 K changes regularly with the increasing of sodium borate concentration. The calculated values of density and refractive index using empirical equations of the ternary system are in good agreement with the experimental values.



Similar content being viewed by others
REFERENCES
T. L. Deng, S. Q. Wang, and Y. F. Guo, Metastable Phase Equilibria and Phase Diagram in Qaidam Basin (Scientific Press, Beijing, 2017).
X. Y. Zheng, M. G. Zhang, Y. Xu, and B. X. Li, Salt Lakes in China (Scientific Press, Beijing, 2002).
M. P. Zheng and X. F. Liu, Acta Geol. Sin. 84, 1585 (2010).
S. Q. Wang, X. M. Du, Y. Jing, et al., J. Chem. Eng. Data 62, 253 (2017).
S. Q. Wang, Y. F. Guo, J. S. Yang, et al., Russ. J. Phys. Chem. A 89, 2190 (2015).
D. C. Li, J. S. Yuan, and S. Q. Wang, Russ. J. Phys. Chem. A 88, 42 (2014).
D. L. Gao, Q. Wang, Y. F. Guo, et al., Fluid Phase Equilib. 371, 121 (2014).
L. Li, Y. F. Guo, S. S. Zhang, et al., Fluid Phase Equilib. 436, 13 (2017).
S. Q. Wang, Y. F. Guo, W. J. Liu, et al., J. Solution Chem. 44, 1545 (2015).
Y. F. Guo, L. Li, L. N. Cao, et al., J. Chem. Eng. Data 62, 508 (2017).
S. Q. Wang, X. N. Han, Y. Jing, et al., J. Chem. Eng. Data 61, 1155 (2016).
S. Q. Wang, F. Y. Guo, D. C. Li, et al., Thermochim. Acta 601, 75 (2015).
Analytical Laboratory of Qinghai Inst. of Salt Lakes at CAS, The Analyses of Brines and Salts, 2nd ed. (Chin. Scientific, Beijing, 1988).
J. D’Ans and K. Behrendt, Kali Steinsalz 2, 126 (1957).
C. H. Fang, J. Salt Lake Res. 2, 15 (1990).
J. M. Speight, Lange’s Handbook of Chemistry, 16th ed. (McGraw-Hill, New York, 2005).
ACKNOWLEDGMENTS
The work was supported by the Program of the National Natural Science Foundation of China (nos. U1507109, U1707602, U1607123, 21773170, and 21106103), the Natural Science Foundation of Tianjin (no. 17JCYBJC19500), the Specialized Research Fund for Doctoral Program of Chinese Higher Education (no. 20111208120003), the Yangtze Scholars and Innovative Research Team in University of Ministry of Education of China (IRT-17R81), and the Innovative Research Team of Tianjin Municipal Education Commission (TD12-5004) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Shi-qiang Wang, Zhao, D., Song, Y. et al. Solubility, Density, and Refractive Index of the Ternary System Na2B4O7 + Mg2B6O11 + H2O at 308.15 K. Russ. J. Phys. Chem. 92, 2601–2605 (2018). https://doi.org/10.1134/S0036024418130289
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418130289