Abstract
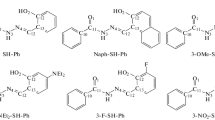
The quinaldinium salt possessing electron withdrawing (-COOEt) groups was condensed with squaric acid giving the quinaldine based symmetrical squaraine dye. Effect of electron withdrawing group on near infrared absorption of the squaraine dye has been studied. Results showed that electron withdrawing group shifts bathochromically the absorption wavelength of squaraine dye by 31 nm when compared with the unsubstituted dye. Computational studies were performed in order to understand the charge transfer and red shift and later have been attributed to the increased biradicaloid character.
Similar content being viewed by others
References
K. Y. Law, Chem. Rev. 93, 449 (1993).
J. Fabian, Chem. Rev. 92, 1197 (1992).
J. Fabian and R. Zahradnik, Angew. Chem. Int. Ed. 28, 677 (1989).
P. V. Kamat, J. Phys. Chem. C 111, 2834 (2007).
K. Takechi, P. V. Kamat, R. R. Avirah, K. Jyothish, and D. Ramaiah, Chem. Mater. 20, 265 (2008).
B. O’Regan, and M. Grätzel, Nature 737, 353 (1991).
T. Geiger, Adv. Funct. Mater. 19, 2720 (2009).
Y. Shi, Angew. Chem. Int. Ed. 50, 6619 (2011).
L. Beverina, D. Ruffo, C. M. Mari, G. A. Pagani, M. Sassi, F. D. Angelis, S. Fantacci, J. H. Yum, M. Gratzel, and M. K. Nazeeruddin, Chem. Sus. Chem. 2, 621 (2009).
U. Mayerhöffer, K. Deing, K. Grub, H. Braunschweig, K. Meerholz, and F. Würthner, Angew. Chem. Int. Ed. 48, 8776 (2009).
IR Absorbing Dyes, Ed. by M. Matsuoka (Plenum, New York, 1990).
Near-Infrared Dyes for High Technology Applications, Ed. by E. Terpetschnig, O. S. Wolfbeis, S. Daehne, U. Resch-Genger, and O. S. Wolfbeis, NATO ASI, Ser. 3: High Technology, Vol. 53 (Kluwer Academic, Dordrecht, 1998).
G. Patonay, J. Salon, J. Sowell, and L. Strekowski, Molecules 9, 40 (2004).
K. Licha and C. Olbrich, Adv. Drug Deliv. Rev. 57, 1087 (2005).
D. G. Devi, T. R. Cibin, D. Ramaiah, and A. Abraham, J. Photochem. Photobiol. B 92, 153 (2008).
M. Umar and W. Relph, Mol. Cancer Ther. 2, 489 (2003).
E. D. Sternberg, D. Dolphin, and C. Bruckner, Tetrahedron 54, 4151 (1998).
G. Seitz and P. Immin, Chem. Rev. 92, 1227 (1992).
A. Ghosh, Chem. Soc. Rev. 32, 181 (2003)
A. Ghosh, Acc. Chem. Res. 38, 449 (2005).
M. Sameiro and T. Gonçalves, Chem. Rev. 109, 190 (2009).
L. Beverina and P. Salice, Eur. J. Org. Chem. 17, 1225 (2010); M. Tian, S. Tatsuura, M. Furuki, Y. Sato, I. Iwasa, and L. S. Pu, J. Am. Chem. Soc. 125, 348 (2003).
S. Tatsuura, M. Tian, M. Furuki, Y. Sato, I. Iwasa, and H. Mitsu, Appl. Phys. Lett. 84, 1450 (2004).
H. Langhals, Angew. Chem. Int. Ed. 42, 4286 (2003).
H. Meier, R. Petermann, and J. Gerold, Chem. Commun., 977 (1999).
K. Yesudas and K. Bhanuprakash, J. Phys. Chem. A 111, 1943 (2007).
Ch. Prabhakar, G. Krishna Chaitanya, S. Sitha, K. Bhanuprakash, and V. Jayathirtha Rao, J. Phys. Chem. A 109, 2614 (2005).
Ch. Prabhakar, K. Yesudas, G. Krishna Chaitanya, S. Sitha, K. Bhanuprakash, and V. Jayathirtha Rao, J. Phys. Chem. A 109, 8604 (2005).
K. Yesudas, G. Krishna Chaitanya, Ch. Prabhakar, K. Bhanuprakash, and V. Jayathritha Rao, J. Phys. Chem. A 110, 11717 (2006).
Ch. Prabhakar, K. Yesudas, K. Bhanuprakash, V. Jayathirtha Rao, R. S. Santosh Kumar, and D. Narayana Rao, J. Phys. Chem. C 112, 13272 (2008).
K. Srinivas, Ch. Prabhakar, C. Lavanya Devi, K. Yesudas, K. Bhanuprakash, and V. Jayathritha Rao, J. Phys. Chem. A 111, 3378 (2007).
A. Thomas, K. Srinivas, Ch. Prabhakar, K. Bhanuprakash, and V. Jayathirtha Rao, Chem. Phys. Lett. 454, 36 (2008).
A. L. Puyad, G. K. Chaitanya, A. Thomas, M. Paramasivam, and K. Bhanuprakash, J. Phys. Org. Chem. 26, 37 (2013).
A. L. Puyad, Ch. Prabhakar, K. Yesudas, K. Bhanuprakash, and V. J. Rao, J. Mol. Struct.: THEOCHEM 904, 1 (2009).
A. L. Puyad, G. K. Chaitanya, Ch. Prabhakar, and K. Bhanuprakash, J. Mol. Model. 19, 275 (2013).
N. Kaila, K. Janz, S. DeBernardo, P. W. Bedard, R. T. Camphausen, S. Tam, D. H. H. Tsao, J. C. Keith, Jr., C. Nicerson-Nutter, A. Shilling, R. Young-Sciame, and Q. Wang, J. Med. Chem. 50, 21 (2007).
N. Kaila, K. Janz, A. Huang, A. Moretto, S. DeBernardo, P. W. Bedard, S. Tam, C. Valerie, J. C. Keith, Jr., D. H. H. Tsao, N. Sushkova, G. D. Shaw, R. T. Camphausen, R. G. Schaub, and Q. Wang, J. Med. Chem. 50, 40 (2007).
M. N. Zemtsova, P. L. Trakhtenberg, and M. V. Galkina, Russ. J. Org. Chem. 39, 1803 (2003).
K. Jyothish, K. T. Arun, and D. Ramaiah, Org. Lett. 6, 3365 (2004).
M. J. Frisch et al., Gaussian 09, Revision C.01 (Gaussian Inc., Wallingford CT, 2009).
R. Bauernschmitt and R. Ahlrichs, J. Chem. Phys. 104, 9047 (1996).
R. Bauernschmitt and R. Ahlrichs, Chem. Phys. Lett. 256, 454 (1996).
J. Wirz, Pure Appl. Chem. 56, 1289 (1984).
M. Nakano, T. Nitta, K. Yamaguchi, B. Champagne, and E. Botek, J. Phys. Chem. A 108, 4105 (2004).
H. Nakatsuji, Acta Chim. Hung., Models Chem. 129, 719 (1992).
H. Nakatsuji, in Computational Chemistry, Reviews of Current Trends, Vol. 2, Ed. by J. Leszczynski (World Scientific, Singapore, 1997).
Avogadro, Version 1.1.0. http://avogadro.openmolecules.net/
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Puyad, A.L., Krishna Chaitanya, G., Karve, S.S. et al. Effect of electron withdrawing groups on near infrared absorption of quinaldine-based squaraine dyes. Russ. J. Phys. Chem. 89, 1087–1090 (2015). https://doi.org/10.1134/S0036024415060047
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024415060047