Abstract
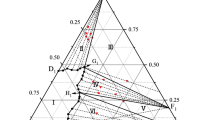
The solubilities in the KCl-MgCl2-H2O system were determined at 50 and 75°C and the phase diagrams and the diagram of refractive index vs composition were plotted. Two invariant point, three univariant curves, and three crystallization zones, corresponding to potassium chloride, hexahydrate (MgCl2 · 6H2O) and double salt (KCl · MgCl2 · 6H2O) showed up in the phase diagrams of the ternary system, The mixing parameters θK, Ca and ΨK, Ca, Cl and equilibrium constant K sp were evaluated in KCl-MgCl2-H2O system by least-squares optimization procedure, in which the single-salt Pitzer parameters of KCl and MgCl2 β(0), β(1), β(2), and C ϕ were directly calculated from the literature. The results obtained were in good agreement with the experimental data.
Similar content being viewed by others
References
T. Raatikainen and A. Laaksonen, Atmos. Chem. Phys. 5, 2475 (2005).
L. Yuanbing and P. George, J. Chem. Eng. Data 49, 1263 (2004).
K. S. Pitzer, J. Phys. Chem. 77, 268 (1973).
C. E. Harvie and J. H. Weare, Geochim. Cosmochim. Acta 44, 981 (1980).
C. E. Harvie, H. P Eugster, and J. H. Weare, Geochim. Cosmochim. Acta 46, 1603 (1982).
C. E. Harvie, N. Moller, and J. H. Weare, Geochim. Cosmochim. Acta 48, 723 (1984).
A. R. Felmy and J. H. Weare, Geochim. Cosmochim. Acta 50, 2771 (1986).
N. Moller, Geochim. Cosmochim. Acta 52, 821 (1988).
R. T. Pabalan and K. S. Pitzer, Geochim. Cosmochim. Acta 51, 2429 (1987).
R. T. Spencer and N. Moller, Geochim. Cosmochim. Acta 54, 575 (1990).
C. F. Purutton and O. F. Tower, J. Am. Chem. Soc. 54, 3040 (1932).
P. S. Song and Y. Yao, CALPHAD 25, 329 (2001).
P. S. Song and Y. Yao, CALPHAD 27, 342 (2003).
Y. H. Li, P. S. Song, S. P. Xia, and S. Y. Gao, CALPHAD 24, 295 (2000).
Y. H. Li, P. S. Song, S. P. Xia, W. Li, and S. Y. Gao, Chin. J. Chem. 23, 953 (2005).
Y. H. Li, P. S. Song, S. P. Xia, W. Li, and S. Y. Gao, CALPHAD 30, 61 (2006).
T. Y. Park, J. Kor. Chem. Soc. 53, 453 (2009).
C. Christov, Geochim. Cosmochim. Acta 71, 3557 (2007).
J. M. Yang, G. Y. Hou, T. R. Ding, and P. Kou, J. Kor. Chem. Soc. 54, 269 (2010).
J. M. Yang and J. Ji, Russian J. Phys. Chem. A 84, 1169 (2010).
D. W. Zeng, W. F. Xu, W. Voigt, and X. Yin, J. Chem. Thermodynamics 40, 1157 (2008).
Qinghai Institute of Salt Lakes of CAS, Analytical Methods of Brines and Salts (Science Press, Beijing, 1988), 2nd ed., p. 39.
H. M. Holmes and R. E. Mesmer, J. Solution Chem. 12, 187 (1983).
M.C.P. de Lima and K. S. Pitzer, Chem. Eng. Data. 30, 120 (1985).
J. A. Rard, R. F. Platford, and K. S. Pitzer, Activity Coefficients in Electrolyte Solutions (CRC Press, Boca Raton, FL, 1991), 2nd ed., p. 75.
C. Balarew, S. Tepavitcharova, and W. Voigt, J. Solution Chem. 30, 815 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Yang, Jm., Peng, J., Duan, Yx. et al. The phase diagrams and Pitzer model representations for the system KCl + MgCl2 + H2O at 50 and 75°C. Russ. J. Phys. Chem. 86, 1930–1935 (2012). https://doi.org/10.1134/S0036024412130146
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024412130146