Abstract
In this work, the cobalt(II) complexation with 1,10-phenanthroline (Phen) in the presence of the [trans-B20H18]2– anion has been studied. At a Co : Phen = 1 : 2 ratio in acetonitrile, a binuclear cobalt(II) complex with bridging chlorine atoms and the boron cluster anion as a counterion [(Phen)2Co(µ-Cl)2Co(Phen)2][trans-B20H18] has been isolated. However, during slow crystallization (within a month), spontaneous isomerization of [trans-B20H18]2– into [iso-B20H18]2– is observed. It has been established by X-ray diffraction analysis that in the crystal of the final compound [(Phen)2Co(µ-Cl)2Co(Phen)2][trans-B20H18]1/3[iso-B20H18]2/3, co-crystallization of both isomeric forms of the octadecahydroeicosaborate anion is observed for the first time. The presence of the iso form of the boron cluster anion is also confirmed by IR spectroscopy data: the spectrum of the product shows a band of stretching vibrations of the BHB bridge groups at 1773 cm–1, which is absent in the trans-form of the boron cluster.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
For many years, the coordination chemistry of polyhedral boron cluster anions [BnHn]2– (n = 6–12) [1–5] has been the subject of intensive research. From the positions of the principle of “hard” and “soft” acids and bases formulated by Pearson, boron cluster anions can be classified as soft bases, which explains the formation of a large number of complexes with metals that are soft acids (copper(I), silver(I), lead(II), cadmium(II)), in which they form the inner coordination sphere of the metal [6–10]. At the same time, with metals that act as intermediate Pearson acids (zinc(II), iron(II), cobalt(II), nickel(II), etc.), boron cluster anions play the role of counterions [11–13], and in the case of some metals that are hard Pearson acids (iron(III), cobalt(III), etc.), the closo-borate anions, as a rule, participate in redox reactions, reducing the oxidation state of the metal [11].
Boron cluster anions possess three-dimensional aromaticity and have delocalized electron density [14, 15], which makes it possible to replace terminal hydrogen atoms with various functional groups [16–20]. Substituted derivatives of boron cluster anions are also capable of forming complexes with metal atoms, acting as ligands of the inner sphere (due to the coordination of B–H groups by the metal atom or due to the coordination of the functional group of the introduced substituent) or as counterions [21–24].
The macropolyhedral dimeric boron cluster anion [trans-B20H18]2– is easily formed upon mild oxidation of the closo-decaborate anion in the presence of iron(III) or cerium(IV) salts in an aqueous solution [25–28] or electrochemical oxidation [29]. The coordination ability of the dimeric cluster is less studied than that of [BnHn]2– boron clusters (n = 10, 12). A number of mixed-ligand complexes of silver with Ph3P and lead(II) complexes with Bipy, which contain a coordinated boron cluster anion, have been obtained [30–32]. In addition, the structures of tris-chelate complexes of manganese(II), iron(II), cobalt(II), and nickel(II) [MnL3][trans-B20H18] (L = Bipy, Phen) [33–35] and iron(II) complex with cyclopentadienyl ligand [CpFe(Cp-CH2-NMe2Et)]2[trans-B20H18] [36] are known; in all these compounds, the boron cluster is located in the outer sphere of the metal.
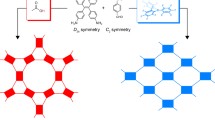
It is known that in solutions of salts of the [trans-B20H18]2– anion in acetonitrile, under the action of UV irradiation, the boron cluster anion transforms into the [iso-B20H18]2– isomer, and when the solution is heated, the reverse process occurs [37–41] (Scheme 1).
It was shown [42] that the [trans-B20H18]2– → [iso-B20H18]2– isomerization process can also proceed without UV irradiation: slow (~1 month) crystallization of [Ag2(Ph3P)6[trans-B20H18]] from DMF leads to the formation of the isomeric complex [Ag2(Ph3P)6[iso-B20H18]].

Scheme 1 .
Solid-phase reversible isomerization [trans-B20H18]2– ↔ [iso-B20H18]2– was found in crystals of silver(I) complexes with triphenylphosphine and lead(II) complexes with 2,2'-bipyridine [30, 31].
In this work, the cobalt(II) chloride complexation with 1,10-phenanthroline in acetonitrile in the presence of the [trans-B20H18]2– anion was studied, and the possibility of spontaneous isomerization of [trans-B20H18]2– → [iso-B20H18]2– in the absence of UV irradiation was found.
EXPERIMENTAL
All reactions were carried out in air. Acetonitrile (HPLC grade), anhydrous CoCl2 (98.0%), and anhydrous Phen (98%) (Sigma-Aldrich) were used without additional purification. (Et3NH)2[B10H10] was synthesized from decaborane-14 according to the known procedure [43]. (Et3NH)2[trans-B20H18] was obtained by mild oxidation of the closo-decaborate anion with an aqueous solution of FeCl3 according to the procedure described [25].
Synthesis of [(Phen)2Co(µ-Cl)2Co(Phen)2][trans-B20H18] (1). CoCl2 (1.2 mmol) was dissolved in acetonitrile (15 mL), a solution of (Et3NH)2[trans-B20H18] (1.2 mmol) in acetonitrile (10 mL) was added, and a twofold excess of Phen (3.6 mmol) in the same solvent (10 mL) was added to the resulting solution. The reaction solution turned pink and crystals of the corresponding color began to form. After 5 h, the colored crystals were filtered off and dried in air. Yield, 77%.
For C48H50N8Co2B20Cl2 anal. calcd. (%): C, 50.40; H, 4.41; N, 9.80; B, 18.9.
Found (%): C, 50.26; H, 4.35; N, 9.71; B, 18.2.
IR (cm–1): ν(BH) 2525, 2493; ν(Phen) 1614, 1582, 1447, 1390, 1347, 1330, 1242, 1157, 1005, 872, 725, 690; π(CH) 845, 732.
Synthesis of [(Phen)2Co(µ-Cl)2Co(Phen)2][trans-B20H18]1/3[iso-B20H18]2/3 (2). The reaction was carried out in the same way as described above, but the resulting solution was hermetically sealed to prevent evaporation of the solvent and left in the dark. Pink crystals began to form within 2–3 weeks. A month later, the crystals were filtered off and dried in air. Yield, 63%. Crystal 2·CH3CN was taken directly from the reaction solution.
For C48H50N8Co2B20Cl2 anal. calcd. (%): C, 50.40; H, 4.41; N, 9.80; B, 18.9.
Found (%): C, 50.31; H, 4.30; N, 9.63; B, 18.7.
IR (cm–1): ν(BH) 2530, 2495, ν(BH)ВНВ 1773; ν(Phen) 1615, 1582, 1449, 1391, 1347, 1328, 1245, 1155, 1008, 872, 725, 691; π(CH) 845, 732.
Elemental analysis was performed on a CHNS-3 FA 1108 Elemental Analyzer automatic gas analyzer (Carlo Erba). Determination of boron and cobalt content by the ICP MS method was performed on an iCAP 6300 Duo inductively coupled plasma atomic emission spectrometer. Before analysis, the samples were dried to constant weight.
IR spectra were recorded on an Infralum FT-02 IR Fourier spectrophotometer (SPF AP Lumeks, Russia); Nujol mull (Aldrich), NaCl plates, region 4000–600 cm–1, resolution 1 cm–1.
X-ray powder diffraction was performed on a Bruker D8 Advance X-ray diffractometer at the Center for Collective Use of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences in CuKα radiation in low-background cuvettes with a substrate of an oriented silicon single crystal in the angle range 2θ 5°–80° with a step of 0.01125°. To obtain diffraction patterns, the samples were carefully ground in an agate mortar.
X-ray diffraction. Sets of diffraction reflections were obtained at the Center for Collective Use of the Kurnakov Institute RAS on an automatic diffractometer Bruker SMART APEX2. The structure was solved by a direct method followed by calculation of difference Fourier syntheses. All non-hydrogen atoms of the cations and boron atoms were refined in the anisotropic approximation, while non-hydrogen atoms of the solvent were refined in the isotropic approximation. All hydrogen atoms were refined according to the rider model with thermal parameters Uiso = 1.2Ueq (Uiso) of the corresponding non-hydrogen atom (1.5Uiso for CH3 groups).
When collecting and processing the array of reflections, the APEX2, SAINT, and SADABS programs were used [44]. The structure was solved and refined using the programs of the OLEX2 complex [45].
The main crystallographic data, experimental parameters, and structure refinement characteristics are given in Table 1. Crystallographic data were deposited with the Cambridge Crystallographic Data Center (CCDC no. 2127924).
The Hirschfeld surface analysis was performed using the Crystal Explorer 17.5 program [46]. Donor-acceptor pairs were visualized using standard (high) surface resolution and dnorm surfaces are displayed in a fixed color scale from –0.640 (red) to 0.986 (cyan) a.e.
RESULTS AND DISCUSSION
When studying the complexation reaction of cobalt(II) chloride with 1,10-phenanthroline (Phen), it was found that a threefold excess of the ligand results in the formation of tris-chelate yellow complex [Co(Phen)3]Cl2, a twofold excess of the ligand leads to the formation of symmetrical binuclear complex [(Phen)2Co(µ-Cl)2Co(Phen)2]Cl2 of pink color, and the equimolar ratio of reagents results in asymmetric complex [Cl2Co(µ-Cl)2Co(Phen)2] [46] of blue color (Scheme 2).

Scheme 2 .
When performing the reaction of cobalt(II) complexation in the presence of the [B10H10]2–, [B12H12]2–, and [B10Cl10]2– boron cluster anions, it was found that a symmetric binuclear complex is formed only in the case of the decachloro-closo-decaborate anion [(Phen)2Co(µ-Cl)2Co(Phen)2][B10Cl10], while in the case of two other boron cluster anions, tris-chelate complexes [Co(Phen)3][An] (An = [B10H10]2– or [B12H12]2–) are formed [47].
In the present work, it was found that carrying out a similar reaction in the presence of the octadecahydroeicosaborate anion also leads to the stabilization of the binuclear cobalt complex and its removal from the reaction solution, which can be explained by the large volume of the anion:
The resulting compound 1 was identified by IR spectroscopy and elemental analysis. In addition, the color of the compound (pink) corresponds to the color of binuclear complexes with Cl– and [B10Cl10]2– counterions [47]. The IR spectrum of the compound shows a band of stretching vibrations of BH bonds with maxima at about 2500 cm–1, as well as a complete set of vibrations of coordinated Phen molecules in the region of 1600–700 cm–1.
Previously, when studying the reaction of silver(I) complexation with Ph3P, it was found that the [trans-B20H18]2– anion spontaneously transforms into the iso form with time. Upon interaction of [Ag(Ph3P)3NO3] with the [trans-B20H18]2– anion in DMF, complex [Ag2(Ph3P)6[trans-B20H18]] is formed from concentrated solutions, and in the case of slow (~1 month) crystallization from the dilute solution in DMF, the final product is complex [Ag2(Ph3P)6[iso-B20H18]], in which a complete conversion of the trans-form of the eicosaborate anion to the iso-form is observed.
In this work, the reagents were kept dissolved in acetonitrile for a long time. For this, the reaction solution was hermetically sealed and left in a dark place. A month later, the resulting crystals 2 were filtered off and studied by elemental analysis, IR spectroscopy, and X-ray diffraction.
According to elemental analysis data, crystals 2 correspond to the same formula [(Phen)2Co(µ-Cl)2Co(Phen)2][B20H18]; however, a new band at 1773 cm–1 is observed in the IR spectrum, which indicates the appearance of the BHB bond in the composition of the compound, which is observed in the [iso-B20H18]2– anion. According to powder X-ray diffraction data, compounds 1 and 2 are isostructural.
Crystals of compound 2⋅CH3CN are built of the [trans-B20H18]2– and [iso-B20H18]2– complex anions in a ratio of 1 : 2, binuclear cations [(Phen)2Co(µ-Cl)2Co(Phen)2]2+, and solvate solvent molecules (Fig. 1). The environment of the metal is distorted octahedral. The B–B bond length in anions is consistent with that in the previously described structures [30]. In the monoclinic cells of complex 2⋅CH3CN, anions are located at the inversion center, while cations are located on the twofold axis. Cations and anions form cation-anion layers parallel to plane bc (Fig. 2).
Intermolecular interactions between the [B20H18]2– anions and surrounding solvent molecules and cations were studied by analyzing the Hirschfeld surface of the anion [47–50]. This tool gives a good visual representation of the presence of intermolecular contacts in crystals and their length. The isomeric boron cluster anions [trans-B20H18]2– and [iso-B20H18]2– were studied separately with the construction of a surface for each boron cluster anion.
An analysis of the Hirschfeld surface of anions in the obtained complexes shows that the strongest noncovalent interactions are formed between the hydrogen atoms of the CH and CH3 groups and the edges or faces of the boron cluster anion (Figs. 3 and 4). The red spots on the Hirschfeld surface of the anion show CH···B contacts, the length of which is less than the sum of the van der Waals atomic radii (3.02 Å for the sum B + H). This is probably due to the fact that the electron density in these compounds is localized over the boron core.
Note that in the closo-decaborate anion, according to the calculated data, the negative charges (NBO and NPA methods) are delocalized over boron atoms (near –0.2e per boron atom), while the charge on hydrogen atoms is slightly higher than 0 (near +0.05 e) [48–51].
An analysis of the 2D unfolding of the Hirschfeld surface of the [B20H18]2– anions shows that the BH···HC contacts of the anions account for 81.5–92.4% of the anion surface, the remaining 7.6–15.3% correspond to the (B)H···C contacts (Figs. 3 and 4). Interestingly, the ratio of the BH···HC and (B)H···C contacts for the two isomeric forms of the anion is practically equal in both cases.
The study of the coordination chemistry of cobalt(II) with organic anions expands the understanding of the diversity of structures of mononuclear, dinuclear, and polymeric cobalt complexes, which opens up new possibilities for the synthesis of coordination polymers, MOFs, and compounds with desired properties [52–56].
CONCLUSIONS
Thus, we have studied the complex formation of cobalt(II) chloride with 1,10-phenanthroline in the presence of the octadecahydro-eicosaborate anion [trans-B20H18]2– and found spontaneous isomerization of the [trans-B20H18]2– anion into [iso-В20Н18]2–. According to X-ray diffraction data, both isomeric boron cluster anions co-crystallize in one crystal.
REFERENCES
E. L. Muetterties and W. H. Knoth, Polyhedral Boranes (Dekker, New York, 1968).
N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, 2dn Ed. (Butterworth-Heinemann, 1997).
Boron Science: New Technologies and Applications, Ed. by N. S. Hosmane (CRC Press, 2012).
I. B. Sivaev and V. I. Bregadze, Polyhedral Boron Hydrides in Use: Current Status and Perspectives (Nova Science Publishers, Hauppauge, 2009).
I. B. Sivaev, Chem. Heterocycl. Comp. 53, 638 (2017). https://doi.org/10.1007/s10593-017-2106-9
V. V. Avdeeva, E. A. Malinina, I. B. Sivaev, et al., Crystals 6, 60 (2016). https://doi.org/10.3390/cryst6050060
E. A. Malinina, S. E. Korolenko, A. S. Kubasov, et al., Polyhedron 184, 11456 (2020). https://doi.org/10.1016/j.poly.2020.114566
S. E. Korolenko, E. A. Malinina, V. V. Avdeeva, et al., Polyhedron 194, 114902 (2021). https://doi.org/10.1016/j.poly.2020.114902
V. V. Avdeeva, E. A. Malinina, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 65, 335 (2020). https://doi.org/10.1134/S003602362003002X
S. E. Korolenko, A. S. Kubasov, L. V. Goeva, et al., Inorg. Chim. Acta 527, 120587 (2021). https://doi.org/10.1016/j.ica.2021.120587
V. V. Avdeeva, E. A. Malinina, K. Yu. Zhizhin, et al., Russ. J. Inorg. Chem. 65, 514 (2020). https://doi.org/10.1134/S0036023620040026
E. A. Malinina, V. V. Avdeeva, S. E. Korolenko, et al., Russ. J. Inorg. Chem. 65, 1343 (2020). https://doi.org/10.1134/S0036023620090119
E. A. Malinina, A. V. Vologzhanina, V. V. Avdeeva, et al., Polyhedron 183, 114540 (2020). https://doi.org/10.1016/j.poly.2020.114540
B. R. King, Chem. Rev. 101, 1119 (2001). https://doi.org/10.1021/cr000442t
Z. Chen and R. B. King, Chem. Rev. 105, 3613 (2005). https://doi.org/10.1021/cr0300892
I. B. Sivaev, Russ. J. Inorg. Chem. 66, 1289 (2021). https://doi.org/10.1134/S0036023621090151
A. V. Nelyubin, I. N. Klyukin, A. P. Zhdanov, et al., Russ. J. Inorg. Chem. 66, 139 (2021). https://doi.org/10.1134/S0036023621020133
A. V. Shmal’ko and I. B. Sivaev, Russ. J. Inorg. Chem. 64, 1726 (2019). https://doi.org/10.1134/S0036023619140067
K. Yu. Zhizhin, A. P. Zhdanov, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 55, 2089 (2010). https://doi.org/10.1134/S0036023610140019
I. B. Sivaev, A. V. Prikaznov, and D. Naoufal, Collect. Czech. Chem. Commun. 75, 1149 (2010). https://doi.org/10.1135/cccc2010054
V. V. Avdeeva, G. A. Buzanov, E. A. Malinina, and N. T. Kuznetsov, Crystals 10, 389 (2020). https://doi.org/10.3390/cryst10050389
V. V. Avdeeva, A. V. Vologzhanina, E. A. Ugolkova, et al., J. Solid State Chem. 296, 121989 (2021). https://doi.org/10.1016/j.jssc.2021.121989
E. Yu. Matveev, I. V. Novikov, A. S. Kubasov, et al., Russ. J. Inorg. Chem. 66, 187 (2021). https://doi.org/10.1134/S0036023621020121
E. A. Malinina, S. E. Korolenko, A. P. Zhdanov, et al., J. Cluster Sci. 32, 755 (2020). https://doi.org/10.1007/s10876-020-01840-5
B. L. Chamberland and E. L. Muetterties, Inorg. Chem. 3, 1450 (1964). https://doi.org/10.1021/ic50020a025
M. F. Hawthorne and R. L. Pilling, J. Am. Chem. Soc. 88, 3873 (1966). https://doi.org/10.1021/ja00968a044
M. F. Hawthorne, K. Shelly, and F. Li, Chem. Commun., 547 (2002). https://doi.org/10.1039/B110076A
Z. B. Curtis, C. Young, R. Dickerson, et al., Inorg. Chem. 13, 1760 (1974). https://doi.org/10.1021/ic50137a046
V. V. Voinova, I. N. Klyukin, A. S. Novikov, et al., Russ. J. Inorg. Chem. 66, 295 (2021). https://doi.org/10.1134/S0036023621030190
V. V. Avdeeva, M. I. Buzin, A. O. Dmitrienko, et al., Chem.—Eur. J. 23, 16819 (2017). https://doi.org/10.1002/chem.201703285
V. V. Avdeeva, M. I. Buzin, E. A. Malinina, et al., Cryst-EngComm 17, 8870 (2015). https://doi.org/10.1039/C5CE00859J
V. V. Avdeeva, E. A. Malinina, K. Yu. Zhizhin, et al., J. Struct. Chem. 60, 692 (2019). https://doi.org/10.1134/S0022476619050020
E. A. Il’inchik, T. M. Polyanskaya, M. K. Drozdova, et al., Russ. J. Gen. Chem. 75, 1545 (2005). https://doi.org/10.1007/s11176-005-0464-y
V. V. Avdeeva, E. A. Malinina, L. V. Goeva, and N. T. Kuznetsov, Dokl. Chem. 474, 141 (2017).
V. V. Avdeeva, A. S. Kubasov, S. E. Korolenko, et al., Polyhedron 217, 115740 (2022).
U. Sirivardane, S. S. C. Chu, N. S. Hosmane, et al., Acta Crystallogr., Sect C 45, 333 (1989). https://doi.org/10.1107/S0108270188010716
A. Kaczmarczyk, R. D. Dobrott, and W. N. Lipscomb, Proc. Nat. Acad. Sci. U.S.A. 48, 729 (1962).
M. F. Hawthorne, R. L. Pilling, P. F. Stokely, and P. M. Garrett, J. Am. Chem. Soc. 85, 3704 (1963).
Z. B. Curtis, C. Young, R. Dickerson, and A. Kaczmarczyk, Inorg. Chem. 13, 1760 (1974).
F. Li, K. Shelly, C. B. Knobler, and M. F. Hawthorne, Angew. Chem., Int. Ed. 37, 1865 (1998).
A. Frances-Monerris, I. Tunon, and A. Monari, J. Phys. Chem. Lett. 10, 6202 (2019). https://doi.org/10.1021/acs.jpclett.9b02760
E. O. Firsova, V. V. Avdeeva, V. I. Privalov, et al., Dokl. Chem. 465, 291 (2015). https://doi.org/10.1134/S0012500815120046
H. C. Miller, N. E. Miller, and E. L. Muetterties, J. Am. Chem. Soc. 85, 3885 (1963). https://doi.org/10.1021/ja00906a033
G. M. Sheldrick, Acta Crystallogr., Sect. C 71, 3 (2015).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009).
M. J. Turner, J. J. McKinnon, S. K. Wolff, et al., CrystalExplorer 17.5 (University of Western Australia, Perth, Australia, 2017).
V. V. Avdeeva, A. V. Vologzhanina, L. V. Goeva, et al., Inorg. Chim. Acta 428, 154 (2015). https://doi.org/10.1016/j.ica.2014.12.029
J. J. McKinnon, D. Jayatilaka, and M. A. Spackman, Chem. Commun. 3814 (2007).
I. N. Klyukin, A. S. Novikov, A. P. Zhdanov, et al., Polyhedron 187, 114682 (2020). https://doi.org/10.1016/j.poly.2020.114682
A. S. Kubasov, E. S. Turyshev, I. V. Novikov, et al., Polyhedron 206, 115347 (2021). https://doi.org/10.1016/j.poly.2021.115347
A. S. Kubasov, A. V. Golubev, A. Yu. Bykov, et al., J. Mol. Struct. 1241, 120591 (2021). https://doi.org/10.1016/j.molstruc.2021.130591
Y. P. Li, G. L. Li, L. Y. Xin, et al., Russ. J. Gen. Chem. 91, 1397 (2021). https://doi.org/10.1134/S1070363221070197
S. I. Dorovskikh, P. A. Stabnikov, L. N. Zelenina, et al., Russ. J. Gen. Chem. 91, 1977 (2021). https://doi.org/10.1134/S107036322110008X
N. V. Gubina, A. A. Markarian, D. S. Kolokolov, et al., Russ. J. Gen. Chem. 91, 2118 (2021). https://doi.org/10.1134/S1070363221100224
M. M. Zherebtsova, N. A. Bogachev, M. Y. Skripkin, et al., Russ. J. Gen. Chem. 91, 1794 (2021). https://doi.org/10.1134/S1070363221090206
M. A. Uvarova and S. E. Nefedov, Russ. J. Inorg. Chem. 66, 1660 (2021). https://doi.org/10.1134/S0036023621110218
Funding
The work was carried out within the framework of the State Assignment of the Kurnakov Institute of General and Inorganic Chemistry RAS in the field of fundamental scientific research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
ADDITIONAL INFORMATION
The article was prepared based on the materials of the XXVIII International Chugaev Conference on Coordination Chemistry, Ol’ginka, Tuapse oblast, Russia, October 3–8, 2021.
Additional information
Translated by V. Avdeeva
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Avdeeva, V.V., Kubasov, A.S., Korolenko, S.E. et al. Spontaneous Isomerization [trans-B20H18]2– → [iso-B20H18]2– during Cobalt(II) Complexation with Phenanthroline. Russ. J. Inorg. Chem. 67, 1169–1177 (2022). https://doi.org/10.1134/S0036023622080022
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622080022