Abstract—The synthesis of NiMoO4 hierarchical nanostructures using the hydrothermal method has been studied. The decomposition of NiMoO4·xH2O crystalline hydrate formed during the synthesis has been studied using synchronous thermal analysis upon heating in a stream of air and argon. According to X-ray diffraction as well as scanning and transmission electron microscopies, the proposed conditions allow one to synthesize single-phase nanosized (average CSR size of about 25 ± 2 nm) nickel(II) molybdate, which has a spinel-type monoclinic structure (space group C2/m) without impurity inclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The implementation of efficient storage of electricity is one of the most important tasks in ensuring the sustainable development of modern society. In this regard, significant efforts of scientists and engineers are focused on the development of portable, energy-intensive, environmentally friendly and at the same time inexpensive energy accumulation and storage devices [1–3]. One of the promising candidates in this context are electrochemical capacitors or supercapacitors, which demonstrate a high charge/discharge rate (on the order of several seconds), high cyclic stability, and specific power in a wide range of operating temperatures (from –50 to + 50°C), as well as a higher operational safety and service life compared to lithium-ion batteries [4–6]. The listed advantages provide serious competitive advantages of supercapacitors in such areas as electric transport, development of portable (including flexible and wearable) electronics, creation of non-volatile memory systems, uninterruptible power supplies, etc. [7, 8]. According to the principle of energy storage, supercapacitors can be divided into two types: (i) supercapacitors with an electric double layer, on the surface of which charge accumulates during the adsorption/desorption of ions, and (ii) supercapacitors with a pseudocapacitive effect, in which energy is accumulated not only through the operation of an electric double layer but also due to reversible redox reactions occurring on the surface or in the near-surface region of the active layer of electrodes [9, 10]. Devices of the second type, as a rule, are characterized by a higher specific capacity [11].
The performance of a supercapacitor is also largely determined by the electrode material. The most popular electrode materials for pseudocapacitive supercapacitors are transition metal oxides such as MnO2, Co3O4, NiO, Co(OH)2, and Ni(OH)2 [12–14]. However, in recent years, there has been a growing interest in the study of electrode materials for supercapacitors based on metal molybdates, in particular, NiMoO4, CoMoO4, and MnMoO4, which are characterized by high electrical conductivity and redox activity [15–18]. It should be noted that electrodes based on nickel molybdate make it possible to achieve the highest values of specific capacity among materials of the family of metal molybdates, which is due to the high electrochemical activity of the nickel ion in the composition of NiMoO4 [19, 20]. An additional improvement in the functional properties of the materials of the electrodes of supercapacitors can be achieved through the directed design of their microstructure. According to modern literature data, the production of nanomaterials (powders and coatings) in the form of hierarchical structures makes it possible, as a rule, to significantly increase the target characteristics of not only the final material but also the device being developed as a whole [21–24]. In particular, it was demonstrated that the production of an active electrode material in the form of nanorods [25–27], nanospheres [28–30], nanofibers [31–33], nanosheets [34–36], and nanoflowers [37–39] allows one achieving high values of specific capacity and rate of charge/discharge, as well as increase the service life of the supercapacitor. Analysis of the literature data showed that one of the most popular and convenient methods for producing nanomaterials for creating alternative energy devices in the form of both powders and coatings with a hierarchical organization is the hydrothermal method [17, 40–42]. In this work, as a preliminary stage of synthesis (formation of the embryonic phase), the programmed coprecipitation of nickel and molybdenum hydroxides was carried out; the thus obtained disperse system was further subjected to hydrothermal treatment. The method of programmed coprecipitation, in comparison with the classical technique, makes it possible to carry out an automated synthesis process with a high degree of reproducibility, and also makes it possible to vary the parameters of the deposition process over a wider range [43, 44].
The aim of this work is to study the formation of 1D nickel molybdate nanostructures under hydrothermal conditions, as well as to study the thermal behavior, crystal structure, and morphology of the resulting powder.
EXPERIMENTAL
Nanopowder of composition NiMoO4 was obtained in accordance with the following scheme. A weighed portion of urea (0.362 g) was added with constant stirring to an aqueous solution of salts NiCl2⋅6H2O and (NH4)6Mo7O24⋅4H2O (0.2 mol/L). The reaction mixture was transferred into a steel autoclave with a Teflon insert and subjected to hydrothermal treatment at a temperature of 120°C for 2 h. Then, the system was naturally allowed to cool to 25°C. The separation and washing of the formed dispersed phase with distilled water was carried out by cyclic centrifugation, after which it was dried at 100°C for 3 h. Then, the obtained powder was heat treated (500°C, 2 h) in oxidizing and inert atmospheres under conditions of simultaneous thermal analysis.
Synchronous (TGA/DSC) thermal analysis of the powder obtained during hydrothermal synthesis and subsequent drying at 100°C was carried out using an SDT Q-600 thermal analyzer (controlled heating was carried out in Al2O3 microcrucibles in the temperature range 25–500°C at a rate of 10 K/min with further exposure at 500°C for 2 h in an argon flow and an air flow, the gas flow rate was 250 mL/min, the weight of the sample was 27.3080 mg).
In order to record the IR transmission spectra of the powders, we prepared the corresponding suspensions in Nujol mull, which were then placed in the form of a film between glasses of potassium bromide. Spectral analysis was performed in the wavenumber range of 350–4000 cm–1 (signal accumulation time was 15 s, and the resolution was 1 cm–1) using an Infralum FT-08 IR Fourier spectrometer.
X-ray powder diffraction studies of the obtained powders were performed using a Bruker D8 Advance diffractometer (CuKα radiation, λ = 1.5418 Å, Ni filter, E = 40 keV, I = 40 mA, 2θ = 5°–80°, resolution 0.02°, signal accumulation time at point 0.3 s).
The morphology and elemental composition of the formed oxide powder was studied using scanning electron microscopy on a Carl Zeiss Nvision 40 three-beam workstation equipped with an Oxford Instruments X-MAX 80 energy dispersive microprobe analyzer. The size and shape of the obtained oxide particles were studied by transmission electron microscopy on a Jeol JEM-1011 microscope equipped with an Orius SC1000W digital camera.
RESULTS AND DISCUSSION
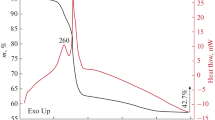
According to the data of synchronous (TGA/DSC) thermal analysis of the powder after drying at 100°C, its behavior in an air flow and in an argon flow is generally similar. Thus, when heated to 500°C, there are three main stages of weight loss: in the ranges 25–190 (4.7%), 190–415 (5.1%), and 415–500°C (2%) (Fig. 1). The first stage (accompanied by a weak endothermic effect) is associated with the removal of the residual solvent, the second stage (the endothermic effect of medium intensity with a minimum at 409°C) is associated with the irreversible process of removal of structurally bound water molecules and the phase transition of NiMoO4⋅xH2O hydrate into oxide, and the third stage, with gradual transformation of the crystal structure of intermediate products and the formation of α-NiMoO4. At the first stage of exposure at 500°C, an insignificant increase in the powder mass was observed, followed by a decrease. As a result, the final weight loss during heat treatment was 11.8%. Thus, based on the data obtained in the course of thermal analysis, the optimal mode of heat treatment (500°C, 2 h, argon or air) of the powder was determined in order to crystallize the target oxide.
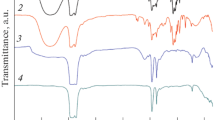
The IR spectra (Fig. 2, spectra 2 and 3) of powders heat-treated at 500°C in a flow of air and in an argon flow show a double absorption band with maxima at 963 and 935 cm–1, as well as bands with maxima at 597 and 450 cm–1, which indicate the formation of a spinel with the α-NiMoO4 structure. At the same time, the absorption bands in the range 920–830 cm–1 observed after drying the powder at 100°C (Fig. 2, spectrum 1) indicate the presence of molybdenum oxide powder [21]. In addition, the powder after drying is characterized by the presence of a more intense absorption band in the region of 3000–3700 cm–1 (stretching vibrations of the OH groups) and an absorption band with a maximum at 1620 cm–1 (bending vibrations of the OH groups) related to the molecules of absorbed water in the structure of the powder, which also indirectly confirms the formation of hydrate NiMoO4⋅xH2O under these conditions. It should be noted that the spectra of nanopowders NiMoO4 obtained upon heat treatment in argon and air are similar, which indicates that the presence of an inert atmosphere is not a prerequisite for the formation of the target structure of α-NiMoO4.
According to the X-ray powder diffraction results (Fig. 3), under the conditions of hydrothermal synthesis followed by drying at 100°C, a crystalline hydrate of the composition NiMoO4⋅xH2O is formed, which agrees with the results of thermal analysis and IR spectroscopy, and further heat treatment at 500°C leads to the formation of single-phase target nickel(II) molybdate with a monoclinic spinel structure (space group C2/m, JCPDS No. 33-0948) [21]. The average CSR size (25 ± 2 nm) of the powder after additional heat treatment at 500°C was calculated using the Scherrer formula: d = Kλ/βcosθ, where d is the average CSR size, K = 0.9 in the approximation that the powder particles have a spherical shape, λ is the X-ray radiation wavelength, β is the reflection width at half maximum, and θ is the diffraction angle. As can be seen from the X-ray powder diffraction pattern, the obtained nanopowder oxide does not contain crystalline impurities of the initial reagents, as well as any intermediate or by-products.
The microstructure of the powder obtained during heat treatment at 500°C was studied using scanning (Fig. 4) and transmission (Fig. 5) electron microscopies. It was determined that the substance has a uniform morphology and consists of organized anisotropic particles, which are layered one-dimensional nanostructures with an average diameter of ~80 nm (the ratio of length to diameter is ~16), consisting of nanorods with a diameter of ~10 nm (the ratio of length to diameter is ~130). Probably, the agglomeration of individual nanorods and the formation of layered 1D structures with two levels of particle organization occurs even at the stage of hydrothermal treatment, preventing of which requires the introduction of appropriate surfactants into the reaction system. The results of microscopy also indicate the absence of impurities that differ in particle shape from the bulk of the substance, while the study of the material in the contrast mode by the average atomic number (reflected electron detector) and the results of the energy dispersive elemental microanalysis confirm the formation of homogeneous nickel(II) molybdate.
In the course of the study, it was shown that the hydrothermal method is an effective method for the formation of layered anisotropic NiMoO4 nanostructures with a monoclinic spinel structure, consisting of nanorods ~10 nm in diameter, and their developed surface makes this approach promising for creating supercapacitor electrodes with a pseudocapacitive effect.
CONCLUSIONS
The process of formation of layered anisotropic nanostructures NiMoO4 with a monoclinic spinel structure under hydrothermal conditions has been studied. According to the data of IR spectroscopy, X‑ray powder diffraction, and thermal analysis, under the conditions of hydrothermal synthesis followed by drying at 100°C, a crystalline hydrate of the composition NiMoO4⋅xH2O is formed, and further heat treatment at 500°C in a flow of air or argon leads to the formation of single-phase nickel molybdate(II) with a monoclinic spinel structure (space group C2/m) and an average CSR size of about 25 ± 2 nm.
Using scanning and transmission electron microscopies, it was shown that the resulting compound has a homogeneous morphology and consists of organized anisotropic particles, which are layered 1D nanostructures with an average diameter of about 80 nm (the ratio of length to diameter is ~16), consisting of nanorods with a diameter of about 10 nm (ratio of length to diameter is ~130). The study of the material in the contrast mode by the average atomic number (reflected electron detector) and the results of the energy dispersive elemental microanalysis confirm the formation of homogeneous nickel(II) molybdate.
Thus, the proposed approach can be recommended for the synthesis of hierarchical nanostructures of the NiMoO4 composition with a developed surface in the creation of functional oxide nanomaterials, including for use in alternative energetics, in particular, in the manufacture of supercapacitor electrodes with a pseudocapacitive effect.
REFERENCES
A. González, E. Goikolea, J. A. Barrena, et al., Renew. Sustain. Energy Rev. 58, 1189 (2016). https://doi.org/10.1016/j.rser.2015.12.249
M. Salanne, B. Rotenberg, K. Naoi, et al., Nat. Energy 1, 16070 (2016). https://doi.org/10.1038/nenergy.2016.70
Y. Shao, M. F. El-Kady, J. Sun, et al., Chem. Rev. 118, 9233 (2018). https://doi.org/10.1021/acs.chemrev.8b00252
S. Rahimi, S. Shahrokhian, and H. Hosseini, J. Electroanal. Chem. 810, 78 (2018). https://doi.org/10.1016/j.jelechem.2018.01.004
C. An, Y. Zhang, H. Guo, et al., Nanoscale Adv. 1, 4644 (2019). https://doi.org/10.1039/c9na00543a
C. Lu and X. Chen, Acc. Chem. Res. 53, 1468 (2020). https://doi.org/10.1021/acs.accounts.0c00205
Y. Wang, Y. Song, and Y. Xia, Chem. Soc. Rev. 45, 5925 (2016). https://doi.org/10.1039/C5CS00580A
L. Li, Z. Lou, D. Chen, et al., Small 14, 1702829 (2018). https://doi.org/10.1002/smll.201702829
Y. Wang, Y. Song, and Y. Xia, Chem. Soc. Rev. 45, 5925 (2016). https://doi.org/10.1039/C5CS00580A
G. Wang, L. Zhang, and J. Zhang, Chem. Soc. Rev. 41, 797 (2012). https://doi.org/10.1039/C1CS15060J
M. Salanne, B. Rotenberg, K. Naoi, et al., Nat. Energy 1, 16070 (2016). https://doi.org/10.1038/nenergy.2016.70
J. Jiang, Y. Li, J. Liu, et al., Adv. Mater. 24, 5166 (2012). https://doi.org/10.1002/adma.201202146
J. Yan, Q. Wang, T. Wei, et al., Adv. Energy Mater. 4, 1300816 (2014).
T. L. Simonenko, N. P. Simonenko, P. Y. Gorobtsov, et al., J. Alloys Compd. 832, 154957 (2020). https://doi.org/10.1016/j.jallcom.2020.154957
T. L. Simonenko, V. A. Bocharova, N. P. Simonenko, et al., Russ. J. Inorg. Chem. 66, 1633 (2021). https://doi.org/10.1134/S0036023621110176
G. K. Veerasubramani, K. Krishnamoorthy, S. Radhakrishnan, et al., Int. J. Hydrogen Energy 39, 5186 (2014). https://doi.org/10.1016/j.ijhydene.2014.01.069
D. Cheng, Y. Yang, J. Xie, et al., J. Mater. Chem. A 3, 14348 (2015). https://doi.org/10.1039/c5ta03455h
D. Cai, D. Wang, B. Liu, et al., ACS Appl. Mater. Interfaces 5, 12905 (2013). https://doi.org/10.1021/am403444v
D. Muthu, S. Vargheese, Y. Haldorai, et al., Mater. Sci. Semicond. Process. 135, 106078 (2021). https://doi.org/10.1016/j.mssp.2021.106078
D. Guo, P. Zhang, H. Zhang, et al., J. Mater. Chem. A 1, 9024 (2013). https://doi.org/10.1039/c3ta11487b
C. Mazzocchia, C. Aboumrad, C. Diagne, et al., Catal. Lett. 10, 181 (1991). https://doi.org/10.1007/BF00772070
V. Augustyn, P. Simon, and B. Dunn, Energy Environ. Sci. 7, 1597 (2014). https://doi.org/10.1039/c3ee44164d
A. Burke, J. Power Sources 91, 37 (2000). https://doi.org/10.1016/S0378-7753(00)00485-7
C. Cui, J. Xu, L. Wang, et al., ACS Appl. Mater. Interfaces 8, 8568 (2016). https://doi.org/10.1021/acsami.6b02962
R. Yang, X. Guo, K. Song, et al., Ceram. Int. 47, 11349 (2021). https://doi.org/10.1016/j.ceramint.2020.12.261
E. R. Ezeigwe, P. S. Khiew, C. W. Siong, et al., Ceram. Int. 43, 13772 (2017). https://doi.org/10.1016/j.ceramint.2017.07.092
S. K. Ray, D. Dhakal, J. K. Sohng, et al., Chem. Eng. J. 347, 366 (2018). https://doi.org/10.1016/j.cej.2018.04.122
Z. Zhang, Y. Liu, Z. Huang, et al., Phys. Chem. Chem. Phys. 17, 20795 (2015). https://doi.org/10.1039/C5CP03331D
D. Cai, D. Wang, B. Liu, et al., ACS Appl. Mater. Interfaces 5, 12905 (2013). https://doi.org/10.1021/am403444v
M. Yao, Z. Hu, Y. Liu, et al., Ionics (Kiel) 22, 701 (2016). https://doi.org/10.1007/s11581-015-1587-8
L. Zhou, S. Zeng, D. Zheng, et al., Chem. Eng. J. 400, 125832 (2020). https://doi.org/10.1016/j.cej.2020.125832
S. Deshagani and D. Maity, A. Das, et al., ACS Appl. Mater. Interfaces 13, 34518 (2021). https://doi.org/10.1021/acsami.1c07064
Y. Zhao, Y. Liu, J. Du, et al., Appl. Surf. Sci. 487, 442 (2019). https://doi.org/10.1016/j.apsusc.2019.05.142
H. Xuan, R. Wang, J. Yang, et al., J. Mater. Sci. 56, 9419 (2021). https://doi.org/10.1007/s10853-021-05902-5
Q. Hu, C. Kang, S. Cao, et al., J. Alloys Compd. 883, 160867 (2021). https://doi.org/10.1016/j.jallcom.2021.160867
Y. Meng, D. Yu, Y. Teng, et al., J. Energy Storage 29, 101195 (2020). https://doi.org/10.1016/j.est.2020.101195
B. Ramulu, S. Chandra Sekhar, G. Nagaraju, et al., Appl. Surf. Sci. 515, 146023 (2020). https://doi.org/10.1016/j.apsusc.2020.146023
C. J. Raj, R. Manikandan, K. H. Yu, et al., Inorg. Chem. Front. 7, 369 (2020). https://doi.org/10.1039/C9QI01085H
A. K. Yedluri, T. Anitha, and H. J. Kim, Energies 12, 1143 (2019).
T. L. Simonenko, V. A. Bocharova, P. Y. Gorobtsov, et al., Russ. J. Inorg. Chem. 65, 1304 (2020). https://doi.org/10.1134/S003602362009018
T. L. Simonenko, V. A. Bocharova, P. Y. Gorobtsov, et al., Russ. J. Inorg. Chem. 65, 1292 (2020). https://doi.org/10.1134/S0036023620090193
Z. Yu, L. Tetard, L. Zhai, et al., Energy Environ. Sci. 8, 702 (2015). https://doi.org/10.1039/c4ee03229b
T. L. Simonenko, N. P. Simonenko, P. Yu, et al., J. Colloid Interface Sci. 588, 209 (2021). https://doi.org/10.1016/j.jcis.2020.12.052
T. L. Simonenko, N. P. Simonenko, E. P. Simonenko, et al., Russ. J. Inorg. Chem. 64, 1475 (2019). https://doi.org/10.1134/S0036023619120167
ACKNOWLEDGMENTS
X-ray powder diffraction and SEM studies were carried out using shared experimental facilities supported by IGIC RAS state assignment.
Funding
This work was partially supported by a Scholarship of the President of the Russian Federation for young scientists and graduate students (project SP-2407.2019.1, synthesis of nanostructures of the NiMoO4 nanopowders) and the Ministry of Science and Higher Education of the Russian Federation within the framework of the State Assignment of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences (X-ray diffraction patterns and SEM studies of the obtained nanopowder).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simonenko, T.L., Bocharova, V.A., Simonenko, N.P. et al. Formation of NiMoO4 Anisotropic Nanostructures under Hydrothermal Conditions. Russ. J. Inorg. Chem. 66, 1779–1784 (2021). https://doi.org/10.1134/S0036023621120160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621120160