Abstract
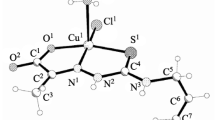
Few novel mixed ligand copper(II) complexes of the type [Cu(L)(Cl)2(H2O)], [Cu(L)2]Cl2, [Cu(L)L1] and [Cu(L)(phen)H2O]Cl2 (where L is the ligand obtained from the condensation of N-(2-aminoethyl)-1,3-propanediamine with m-nitrobenzaldehyde (La)/o-chlorobenzaldehyde (Lb)/benzaldehyde (Lc)/p-methoxybenzaldehyde (Ld)/p-hydroxybenzaldehyde (Le)/furfuraldehyde (Lf)/pyrrole-2-carboxaldehyde (Lg); L1 is another ligand obtained from the condensation of anthranilic acid with salicyaldehyde; phen = 1,10-phenanthroline) have been synthesized and characterized by the spectral and analytical techniques. From these data, it is found that the ligands adopt distorted octahedral geometry on metalation with Cu(II) ion. The XRD data indicate that the complexes are polycrystalline with nanosized grains. The SEM images of [Cu(La)phen(H2O)]Cl2 and [Cu(Lf)2]Cl2 complexes show that they have leaf and cauliflower like morphology. The in vitro biological screening effects of the investigated compounds have been tested against the bacteria such as Escherichia coli, Klebsiella pneumoniae, Salmonella typhi, Pseudomonas aeruginosa and Staphylococcus aureus and fungi such as Aspergillus niger, Rhizopus stolonifer, Aspergillus flavus, Rhizoctonia bataicola and Candida albicans by the well diffusion method. A comparative study of MIC values of the Schiff base ligands and their complexes indicates that the complexes exhibit higher antimicrobial activity than the free ligands. An electrochemical study of the copper complexes containing electron withdrawing substituted ligands reveals that they prefer to bind to DNA in Cu(II) rather than Cu(I) oxidation state.
Similar content being viewed by others
References
A. M. Pyle and J. K. Barton, Prog. Inorg. Chem. 38, 413 (1990).
B. Macias, M. V. Villa, B. Gomez, et al., J. Inorg. Biochem. 101, 444 (2007).
C. P. Raptopoulou, S. Paschalidou, A. A. Pantazaki, et al., J. Inorg. Biochem. 71, 15 (1998).
P. RuÍz, R. Ortiz, L. Perelló, et al., J. Inorg. Biochem. 101, 831 (2007).
C. Liu, M. Wang, T. Zhang, and H. Sun, Coord. Chem. Rev. 248, 147 (2004).
M. Dizdaroglu, Free Radical Biol. Med. 10, 225 (1991).
S. Belaid, A. Landreau, S. Djebbar, et al., J. Inorg. Biochem. 102, 63 (2008).
J. Liu, H. Zhang, C. Chen, et al., Dalton Trans., 114 (2003).
K. J. Humphreys, K. D. Karlin, and S. E. Rokita, J. Am. Chem. Soc. 124, 6009 (2002).
C. A. Detmer, F. V. Pamatong, and J. R. Bocarsly, Inorg. Chem. 36, 3676 (1997).
K. J. Humphreys, A. E. Johnson, K. D. Karlin, and S. E. Rokita, J. Biol. Inorg. Chem. 7, 835 (2002).
S. T. Frey, H. H. Sun, N. N. Murthy, and K. Karlin, D, Inorg. Chim. Acta 242, 329 (1996).
A. Garc’a-Raso, J. J. Fiol, B. Adrover, et al., J. Inorg. Biochem. 95, 77 (2003).
X. Wang, H. Chao, H. Li, et al., J. Inorg. Biochem. 98, 423 (2004).
A. M. Thomas, M. Nethaji, A. R. Chakravarty, J. Inorg. Biochem. 98, 1087 (2004).
T. Hirohama, Y. Kuranuki, E. Ebina, et al., J. Inorg. Biochem. 99, 1205 (2005).
H. Zhang, C. S. Liu, X. H. Bu, M. Yang, J. Inorg. Biochem. 99, 1119 (2005).
J. R. J. Sorenson, J. Med. Chem. 19, 135 (1976).
D. H. Brown, A. J. Lewis, W. E. Smith, J. W. Teape, J. Med. Chem. 23, 729 (1980).
V. K. Sharma, O. P. Pandey, S. K. Sengupta, Trans. Met. Chem. 12, 509 (1987).
R. Reiner, Antibiotics-an introduction, Roche Scientific Services, Switzerland, 1, 21 (1982).
M. E. Reichmann, S. A. Rice, C. A. Thomas, P. Doty, J. Am. Chem. Soc. 76, 3047 (1954).
N. Raman, A. Sakthivel, K. Rajasekaran, J. Coord. Chem. 62, 1661, 2009.
W. J. Geary, Coord. Chem. Rev. 7, 81 (1971).
R. K. Ray, G. R. Kauffman, Inorg. Chim. Acta 173, 207 (1990).
R. J. Dudley, B. J. Hathaway, J. Chem. Soc. A, 2099 (1970).
D. E. Billing, B. J. Hathaway, P. Nicholls, J. Chem. Soc. A, 1877 (1970).
A. K. Das, Medicinal Aspects of Bioinorganic Chemistry (CBS, Shahdara, Delhi, Ch. 3, 1990).
Y. Anjaneyula, R. P. Rao, Synth. React. Inorg. Met.-Org. Chem., 16, 257 (1986).
L. Mishra, K. K. Upadhyay, V. K. Singh, Synth. React. Inorg. Met.-Org. Chem. 26, 23 (1996).
P. Viswanathamurthi, N. Dharmaraj, K. Natarajan, Synth. React. Inorg. Met.-Org. Chem. 30, 1273 (2000).
J. D. Ming, J. D. Epperson, J. Inorg. Biochem. 91, 46 (2002).
M. Z. Wang, Z. X. Meng, B. L. Liu, et al., Inorg. Chem. Commun. 8, 368 (2005).
N. Raman, A. Kulandaisamy, A. Shunmugasundaram, K. Jeyasubramanian, Trans. Met. Chem. 26, 131 (2001).
Z. H. Chohan, Synth.React. Inorg. Met.-Org. Chem. 34, 833 (2004).
Z. H. Chohan, C. T. Supuran, A. Scozzafava, J. Enzym. Inhib. Med. Chem. 19, 79 (2004).
H. M. Butler, A. Hurse, E. Thursky, A. Shulman, Aust. J. Expt. Biol. Med. Sci. 47, 541 (1969).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Raman, N., Joseph, J. Novel metal-based antimicrobial agents of copper(II) complexes: Synthesis, spectral characterization and DNA interaction study. Russ. J. Inorg. Chem. 55, 1064–1074 (2010). https://doi.org/10.1134/S0036023610070120
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023610070120