Abstract
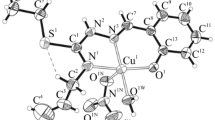
2-Oxopropanoic acid reacts in ethanol with N-(prop-2-en-1-yl)hydrazinecarbothioamide in a 1 : 1 mole ratio to form thiosemicarbazone H2L. Coordination compounds Cu(HL)X [X = Cl–, Br–, NO3–], Cu(H2O)(L), Ni(HL)2, Co(HL)2X [X = Cl–, Br–], and Fe(HL)2X [X = NO3–, Br–] are formed in the reactions of H2L with copper(II), nickel(II), cobalt(II), and iron(III) salts. The reactions of Cu(H2O)(L) with imidazole (Im) and 3,4-dimethylpyridine (3,4-Lut) result in mixed-ligand complexes Cu(A)(L) [A = Im, 3,4-Lut]. The structures of two copper complexes were determined by single crystal X-ray diffraction analysis. The synthesized complexes exhibit selective antimicrobial and antifungal activity in the concentration range of 15.62–1000 μg/mL. The introduction of amines into the inner sphere of copper complexes leads to an increase in the antimicrobial activity.






Similar content being viewed by others
REFERENCES
Beraldo, H. and Gambino, D., Mini Rev. Med. Chem., 2004, vol. 4, no. 1, p. 31. https://doi.org/10.2174/1389557043487484
Gulea, A.P., Graur, V.O., Chumakov, Yu.M., Petrenko, P.A., Balan, G.G., Burduniuc, O.S., Tsapkov, V.I., and Rudic, V.F., Russ. J. Gen. Chem., 2019, vol. 89, no. 5, p. 953. https://doi.org/10.1134/S1070363219050153
Pahontu, E., Fala, V., Gulea, A., Poirier, D., Tapcov, V., and Rosu, T., Molecules, 2013, vol. 18, no. 8, p. 8812. https://doi.org/10.3390/molecules18088812
Lukmantara, A.Y., Kalinowski, D., Kumar, N., and Richardson, D.R., J. Inorg. Biochem., 2014, vol. 141, p. 43. https://doi.org/10.1016/j.jinorgbio.2014.07.020
Diaz, A., Cao, R., and Garcia, A., Monatsh. Chem., 1994, vol. 125, nos. 8–9, p. 823. https://doi.org/10.1007/BF00812694
Pathan, A.H., Bakale, R.P., Naik, G.N., Frampton, C.S., and Gudasi, K.B., Polyhedron, 2012, vol. 34, no. 1, p. 149. https://doi.org/10.1016/j.poly.2011.12.033
Pathan, A.H., Ramesh, A.K., Bakale, R.P., Naik, G.N., Rohit Kumar, H.G., Frampton, C.S., Advi Rao, G.M., and Gudasi, K.B., Inorg. Chim. Acta, 2015, vol. 430, p. 216. https://doi.org/10.1016/j.ica.2015.03.013
Baldini, M., Belicchi-Ferrari, M., Bisceglie, F., Dall’Aglio, P. P., Pelosi, G., Pinelli, S., and Tarasconi, P., Inorg. Chem., 2004, vol. 43, no. 22. P. 7170. https://doi.org/10.1021/ic049883b
Pelosi, G., Open Crystallogr. J., 2010, vol. 3, p. 16. https://doi.org/10.2174/1874846501003010016
Prisakar’, V.I., Tsapkov, V.I., Buracheeva, S.A., Byrke, M.S., and Gulya, A.P., Pharm. Chem. J., 2005, vol. 39, no. 6, p. 313. https://doi.org/10.1007/s11094-005-0142-8
Samus’, N.M., Gulya, A.P., Tsapkov, V.I., Chumakov, Y.M., and Roshu, T., Russ. J. Gen. Chem., 2006, vol. 76, no. 7, p. 1100. https://doi.org/10.1134/s1070363206070164
Belicchi-Ferrari, M., Bisceglie, F., Buluggiu, E., Pelosi, G., and Tarasconi, P., Polyhedron, 2010, vol. 29, no. 10, p. 2134. https://doi.org/10.1016/j.poly.2010.04.009
Belicchi-Ferrari, M., Bisceglie, F., Buluggiu, E., Pelosi, G., and Tarasconi, P., Polyhedron, 2009, vol. 28, no. 6, p. 1160. https://doi.org/10.1016/j.poly.2009.01.013
Allen, F.H., Acta Crystallogr. B, 2002, vol. 58, p. 380. https://doi.org/10.1107/S0108768102003890
CrysAlis RED, Oxford Diffraction Ltd., Version 1.171.34.76, 2003.
Sheldrich, G.M., Acta Crystallogr. (А), 2008, vol. 64, p. 112. https://doi.org/10.1107/S0108767307043930
Gulea, A., Poirier, D., Roy, J., Stavila, V., Bulimestru, I., Tapcov, V., and Popovschi, L, J. Enzyme Inhib. Med. Chem., 2008, vol. 23, no. 6, p. 806. https://doi.org/10.1080/14756360701743002
Pahontu, E., Usataia, I., Graur, V., Chumakov, Yu., Petrenko, P., Gudumac, V., and Gulea, A., Appl. Organometal. Chem., 2018, vol. 32, no. 12, p. e4544. https://doi.org/10.1002/aoc.4544
Funding
The work was carried out within the framework of the State program of the Republic of Moldova (project 20.80009.5007.10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Gulea, А.P., Graur, V.О., Diurici, E.C. et al. Synthesis, Structure, and Biological Activity of Copper(II), Nickel(II), Cobalt(III), and Iron(III) Coordination Compounds with 2-{2-[(Prop-2-en-1-yl)carbamothioyl]hydrazinylidene}propanoic Acid. Russ J Gen Chem 90, 2120–2127 (2020). https://doi.org/10.1134/S107036322011016X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036322011016X