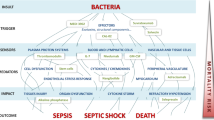
Abstract
Sepsis is a generalized infection accompanied by response of the body that manifests in a clinical and laboratory syndrome, namely, in the systemic inflammatory response syndrome (SIRS) from the organism to the infection. Although sepsis is a widespread and life-threatening disease, the assortment of drugs for its treatment is mostly limited by antibiotics. Therefore, the search for new cellular targets for drug therapy of sepsis is an urgent task of modern medicine and pharmacology. One of the most promising targets is the adenosine A2A receptor (A2AAR). The activation of this receptor, which is mediated by extracellular adenosine, manifests in almost all types of immune cells (lymphocytes, monocytes, macrophages, and dendritic cells) and results in reducing the severity of inflammation and reperfusion injury in various tissues. The activation of adenosine A2A receptor inhibits the proliferation of T cells and production of proinflammatory cytokines, which contributes to the activation of the synthesis of anti-inflammatory cytokines, thereby suppressing the systemic response. For this reason, various selective A2AAR agonists and antagonists may be considered to be drug candidates for sepsis pharmacotherapy. Nevertheless, they remain only efficient ligands and objects of pre-clinical and clinical trials. This review examines the molecular mechanisms of inflammatory response in sepsis and the structure and functions of A2AAR and its role in the pathogenesis of sepsis, as well as examples of using agonists and antagonists of this receptor for the treatment of SIRS and sepsis.
Similar content being viewed by others
Abbreviations
- MOF:
-
multiple organ failure
- 5-LO:
-
5-lipoxigenase
- A2AAR:
-
adenosine A2A receptor
- AC:
-
adenylate cyclase
- AP-1:
-
activator protein 1
- AR:
-
adenosine receptor
- CD:
-
cluster of differentiation (of lymphocytes)
- CDC42:
-
cell division control protein 42 homolog
- CEBPβ:
-
CCAAT-enhancer-binding protein
- beta-COX-2:
-
cycloxygenase of type 2
- CREB1:
-
cAMP responsive element binding protein 1
- CTLA-4:
-
cytotoxic T-lymphocyte-associated antigen 4
- CXCL:
-
chemokine (C-X-C motif) ligand 1
- eNOS:
-
endothelial NO synthase
- GCSF:
-
granulocyte colony-stimulating factor
- GPCR:
-
G-protein-coupled receptor
- ICAM-1:
-
intercellular adhesion molecule 1
- IFN:
-
interferon
- IL:
-
interleukin
- iNOS:
-
inducible NO synthase
- IP3:
-
inositol 3,4,5-triphosphate
- MAPK:
-
mitogen-activated protein kinase
- MCSF:
-
macrophage colony-stimulating factor
- MOF:
-
multiple organ failure
- NF-κB:
-
nuclear factor κB
- NOS:
-
NO synthase
- PAF:
-
platelet activating factor
- PAI-1:
-
plasminogen activator inhibitor-1
- PDZ-GEF1:
-
guanine nucleotide exchange factor containing PDZ-domain
- PGE2:
-
prostaglandin E2
- PI3K:
-
phosphoinositide 3-kinase
- PIP2:
-
phosphatidylinositol 4,5-diphosphate
- PKA:
-
protein kinase A
- PKB (or Akt):
-
protein kinase B
- PKC-zeta:
-
protein kinase С-zeta
- SIRS:
-
systemic inflammatory response syndrome
- SUMO-1:
-
small ubiquitin-related modifier 1
- TF:
-
tissue factor III (thromboplastin)
- TGF-β:
-
transforming growth factor beta
- TLRs:
-
Toll-like receptors
- TNF-α:
-
tumor necrosis factor α
- TRAX:
-
translin-associated factor X
- USP4:
-
ubiquitin specific protease 4
References
Haskó G., Linden J., Cronstein B., Pacher P. 2008. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 7, 759–770.
Jacobson K.A., Gao Z.-G. 2006. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 5, 247–264.
Anderson C.M., Xiong W., Geiger J.D., Young J.D., Cass C.E., Baldwin S.A., Parkinson F.E. 1999. Distribution of equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporters (ENT1) in brain. J. Neurochem. 73, 867–873.
Fredholm B.B., Arslan G., Halldner L., Kull B., Schulte G., Wasserman W. 2000. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 362, 364–374.
Chen J.-F., Eltzschig H.K., Fredholm B.B. 2013. Adenosine receptors as drug targets: What are the challenges? Nat. Rev. Drug Discov. 12, 265–286.
Morelli M., Carta A.R., Jenner P. 2009. Adenosine receptors in health and disease. Handb. Exp. Pharmacol. 193, 589–615.
Van der Poll T., Opal S.M. 2008. Host–pathogen interactions in sepsis. Lancet Infect. Dis. 8, 32–43.
Cohen J. 2002. The immunopathogenesis of sepsis. Nature. 420, 885–891.
Stearns-Kurosawa D.J., Osuchowski M.F., Valentine C., Kurosawa S., Remick D.G. 2011. The pathogenesis of sepsis. Annu. Rev. Pathol. 6, 19–48.
Reinhart K., Bauer M., Riedemann N.C., Hartog C.S. 2012. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin. Microbiol. Rev. 25, 609–634.
Ohta A., Sitkovsky M. 2001. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 414, 916–920.
Grinev M.V., Gromov M.I., Komrakov V.E. 2001. Khirurgicheskii sepsis (Surgical Sepsis). St. Petersburg.
Savel’ev V.S., Gel’fand B.R. 2010. Sepsis: klassifikatsiya, klinikodiagnosticheskaya kontseptsiya i lechenie (Sepsis: Claccification, Clinical Diagnostic Concept, and Treatment). Moscow: Med. Inform. Agentstvo.
Lobzin Yu.V., Kozhokaru D.I. 2012. Intensive treatment of severe influenza compliucations. Zh. Infektol. 4, 58–64.
Chuchalin A.G, Sologub T.V. (Eds.). 2014. Gripp u vzroslykh: metodicheskie rekomendatsii po diagnostike, lecheniyu, spetsificheskoi i nespetsificheskoi profilaktike Influenza in Adults: Methodologica Guidelines for Diagnosis, Treatment, and Specific and Nonspecific Prevention). St. Petersburg: NO-Print.
Kiselev O.I., Vasin A.V., Shevyreva M.P., Deeva E.G., Sivak K.V., Egorov V.V., Tsvetkov V.B., Egorov A.Yu., Romanovskaya-Romanko E.A., Stepanova L.A., Komissarov A.B., Tsybalova L.M., Ignatjev G.M. 2015. Ebola hemorrhagic fever: Properties of the pathogen and development of vaccines and chemotherapeutic agents. Mol. Biol. (Moscow). 49 (4), 480–493.
Liew F.Y., Xu D., Brint E.K., O’Neill L.A. 2005. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458.
Salomao R., Brunialti M.K.C., Rapozo M.M., Baggio-Zappia G.L., Galanos C., Freudenberg M. 2012. Bacterial sensing, cell signaling, and modulation of the immune responsed during sepsis. Shock. 38, 227–242.
Lopez-Bojorquez L.N., Lopez-Bojorquez L.N., Dehesa A.Z., Dehesa A.Z., Reyes-Teran G., Reyes-Teran G. 2004. Molecular mechanisms involved in the pathogenesis of septic shock. Arch. Med. Res. 35, 465–479.
Liu S.F., Malik A.B. 2006. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L622–L645.
Wen A.Y., Sakamoto K.M., Miller L.S. 2010. The role of the transcription factor CREB in immune function. J. Immunol. 185, 6413–6419.
Sullivan G.W., Fang G., Linden J., Scheld W.M. 2004. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J. Infect. Dis. 189, 1897–1904.
Sheth S., Brito R., Mukherjea D., Rybak L.P., Ramkumar V. 2014. Adenosine receptors: Expression, function and regulation. Int. J. Mol. Sci. 15, 2024–2052.
Lebon G., Warne T., Edwards P.C., Bennett K., Langmead C.J., Leslie A.G.W., Tate C.G. 2011. Agonistbound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 474, 521–525.
Doré A.S., Robertson N., Errey J.C., Ng I., Hollenstein K., Tehan B., Hurrell E., Bennett K., Congreve M., Magnani F., Tate C.G., Weir M., Marshall F.H. 2011. Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure. 19, 1283–1293.
Xu F., Wu H., Katritch V., Han G.W., Jacobson K.A., Gao Z.-G., Cherezov V., Stevens R.C. 2011. Structure of an agonist-bound human A2A adenosine receptor. Science. 332, 322–327.
Preti D., Baraldi P.G., Moorman A.R., Borea P.A., Varani K. 2015. History and perspectives of A2A adenosine receptor antagonists as potential therapeutic agents. Med. Res. Rev. 35, 790–848.
Muravleva L.E., Molotov-Luchanskii V.B., Klyuev D.A., Ponamareva O.A., Demidchik L.A., Kolesnikova E.A. 2012. On the role of adenosine in mechanisms of development and progression of lung diseases. Sovr. Probl. Nauki Obraz. 4, 25.
Awad A.S., Huang L., Ye H., Duong E.T.A., Bolton W.K., Linden J., Okusa M.D. 2006. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 290, F828–F837.
Reutershan J., Cagnina R.E., Chang D., Linden J., Ley K. 2007. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J. Immunol. 179, 1254–1263.
Trevethick M.A., Mantell S.J., Stuart E.F., Barnard A., Wright K.N., Yeadon M. 2008. Treating lung inflammation with agonists of the adenosine A2A receptor: Promises, problems and potential solutions. Br. J. Pharmacol. 155, 463–474.
Klinger M., Freissmuth M., Nanoff C. 2002. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell. Signal. 14, 99–108.
De Lera Ruiz M., Lim Y.-H., Zheng J. 2014. Adenosine A2A receptor as a drug discovery target. J. Med. Chem. 57, 3623–3650.
Schulte G., Fredholm B.B. 2003. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell. Signal. 15, 813–827.
Kasza A. 2013. Signal-dependent Elk-1 target genes involved in transcript processing and cell migration. Biochim. Biophys. Acta Gene Regul. Mech. 1829, 1026–1033.
Delghandi M.P., Johannessen M., Moens U. 2005. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell. Signal. 17, 1343–1351.
Feoktistov I., Goldstein A.E., Biaggioni I. 2000. Cyclic AMP and protein kinase A stimulate Cdc42: Role of A(2) adenosine receptors in human mast cells. Mol. Pharmacol. 58, 903–910.
Qiu R.G., Abo A., Steven Martin G. 2000. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr. Biol. 10, 697–707.
Yang E., Zha J., Jockel J., Boise L.H., Thompson C.B., Korsmeyer S.J. 1995. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 80, 285–291.
Hayakawa J., Mittal S., Wang Y., Korkmaz K.S., Adamson E., English C., e Ohmichi M., McClelland M., Mercola D. 2004. Identification of promoters bound by c-Jun/ATF2 during rapid large-scale gene activation following genotoxic stress. Mol. Cell. 16, 521–535.
Yu T., Li Y.J., Bian A.H., Zuo H.B., Zhu T.W., Ji S.X., Kong F., Yin de Q., Wang C.B., Wang Z.F., Wang H.Q., Yang Y., Yoo B.C., Cho J.Y. 2014. The regulatory role of activating transcription factor 2 in inflammation. Mediators Inflamm. 2014, 950472. doi 10.1155/2014/ 950472
Costa C., Hirsch E. 2010. More than just kinases: The scaffolding function of PI3K. Curr. Top. Microbiol. Immunol. 346, 171–181.
Katso R., Okkenhaug K., Ahmadi K., White S., Timms J., Waterfield M.D. 2001. Cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 17, 615–675.
Burgering B.M., Coffer P.J. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 376, 599–602.
McColl S.R., St-Onge M., Dussault A.-A., Laflamme C., Bouchard L., Boulanger J., Pouliot M. 2006. Immunomodulatory impact of the A2A adenosine receptor on the profile of chemokines produced by neutrophils. FASEB J. 20, 187–189.
Folkesson H.G., Kuzenko S.R., Lipson D.A., Matthay M.A., Simmons M.A. 2012. The adenosine 2A receptor agonist GW328267C improves lung function after acute lung injury in rats. AJP Lung Cell. Mol. Physiol. 303, L259–L271.
Moore C.C., Martin E.N., Lee G.H., Obrig T., Linden J., Scheld W.M. 2008. An A2A adenosine receptor agonist, ATL313, reduces inflammation and improves survival in murine sepsis models. BMC Infect. Dis. 8, 141.
Cavalcante I.C., Castro M.V., Barreto A.R.F., Sullivan G.W., Vale M., Almeida P.R.C., Linden J., Rieger J.M., Cunha F.Q., Guerrant R.L., Ribeiro R.A., Brito G.A. 2006. Effect of novel A2A adenosine receptor agonist ATL 313 on Clostridium difficile toxin A-induced murine ileal enteritis. Infect. Immun. 74, 2606–2612.
Savel’ev V.S., Gel’fand B.R. 2006. Sepsis v nachale XXI veka. Klassifikatsiya, kliniko-diagnosticheskaya kontseptsiya i lechenie. Patologo-anatomicheskaya diagnostika (Sepsis in the Early 21st Century: Classification, Clinical Diagnostic Concept and Treatment, and Pathoanatomical Diagnosis). Moscow: Litterra.
Swaminathan S., Rosner M.H., Okusa M.D. 2015. Emerging therapeutic targets of sepsis-associated acute kidney injury. Semin. Nephrol. 35, 38–54.
Savva A., Roger T. 2013. Targeting Toll-like receptors: Promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front. Immunol. 4, 387. doi 10.3389/fimmu.2013.00387
Rusinov V.L., Sapozhnikova I.M., Ulomskii E.N., Medvedeva N.R., Egorov V.V., Kiselev O.I. 2015. Nucleophilic substitution of nitro group in nitrotriazolotriazines as a model of potential interaction with cysteinecontaining proteins. Chem. Heterocycl. Compd. 51, 275–280.
Liu Q., Li J., Khoury J., Colgan S.P., Ibla J.C. 2009. Adenosine signaling mediates SUMO-1 modification of IkappaBalpha during hypoxia and reoxygenation. J. Biol. Chem. 284 (20), 13686–13695. doi 10.1074/jbc
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © K.V. Sivak, A.V. Vasin, V.V. Egorov, V.B. Tsevtkov, N.N. Kuzmich, V.A. Savina, O.I. Kiselev, 2016, published in Molekulyarnaya Biologiya, 2016, Vol. 50, No. 2, pp. 231–245.
Rights and permissions
About this article
Cite this article
Sivak, K.V., Vasin, A.V., Egorov, V.V. et al. Adenosine A2A receptor as a drug target for treatment of sepsis. Mol Biol 50, 200–212 (2016). https://doi.org/10.1134/S0026893316020230
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893316020230