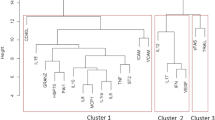
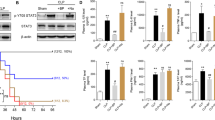
Abstract
Sepsis is a life-threatening organ dysfunction caused by a dysregulated inflammatory response to infection. To date, there is no specific treatment established for sepsis. In the extracellular compartment, purines such as adenosine triphosphate (ATP) and adenosine play essential roles in the immune/inflammatory responses during sepsis and septic shock. The balance of extracellular levels among ATP and adenosine is intimately involved in the signals related to immune stimulation/immunosuppression balance. Specialized enzymes, including CD39, CD73, and adenosine deaminase (ADA), are responsible to metabolize ATP to adenosine which will further sensitize the P2 and P1 purinoceptors, respectively. Disruption of the purinergic pathway had been described in the sepsis pathophysiology. Although purinergic signaling has been suggested as a potential target for sepsis treatment, the majority of data available were obtained using pre-clinical approaches. We hypothesized that, as a reflection of deregulation on purinergic signaling, septic patients exhibit differential measurements of serum, neutrophils and monocytes purinergic pathway markers when compared to two types of controls (healthy and ward). It was observed that ATP and ADP serum levels were increased in septic patients, as well as the A2a mRNA expression in neutrophils and monocytes. Both ATPase/ADPase activities were increased during sepsis. Serum ATP and ADP levels, and both ATPase and ADPase activities were associated with the diagnosis of sepsis, representing potential biomarkers candidates. In conclusion, our results advance the translation of purinergic signaling from pre-clinical models into the clinical setting opening opportunities for so much needed new strategies for sepsis and septic shock diagnostics and treatment.




Similar content being viewed by others
References
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet Infect Dis. 2008;8(1):32–43.
Medeiros PMC, Schjalm C, Christiansen D, et al. Vitamin C, hydrocortisone, and the combination thereof significantly inhibited two of nine inflammatory markers induced by escherichia coli but not by staphylococcus aureus - when incubated in human whole blood. Shock. 2022;57(1):72–80.
Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87.
Haas CB, Lovaszi M, Braganhol E, Pacher P, Hasko G. Ectonucleotidases in inflammation, immunity, and cancer. J Immunol. 2021;206(9):1983–90.
Lovaszi M, Branco Haas C, Antonioli L, Pacher P, Hasko G. The role of P2Y receptors in regulating immunity and metabolism. Biochem Pharmacol. 2021;187:114419.
Haas CB, Lovaszi M, Pacher P, et al. Extracellular ectonucleotidases are differentially regulated in murine tissues and human polymorphonuclear leukocytes during sepsis and inflammation. Purinergic Signal. 2021;17(4):713–24.
Hasko G, Csoka B, Koscso B, et al. Ecto-5’-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J Immunol. 2011;87(8):4256–67.
Nemeth ZH, Csoka B, Wilmanski J, et al. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176(9):5616–26.
Di Virgilio F, Sarti AC, Coutinho-Silva R. Purinergic signaling DAMPs and inflammation. Am J Physiol Cell Physiol. 2020;318(5):C832–5. https://doi.org/10.1152/ajpcell.00053.2020.
Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2013;368(13):1260.
Sevigny J, Martin-Satue M, Pintor J. Purinergic Signalling in Immune System Regulation in Health and Disease. Mediators Inflamm. 2015;2015(1):106863.
Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31. https://doi.org/10.1016/j.immuni.2017.06.020.
Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
project TBI. Brazilian National Intensive Care Register. Comorbidities. www.utisbrasileiras.com.br/en/icu-adult/comorbidades. Published 2022. Accessed 02/04, 2022.
Oh H, Siano B, Diamond S. Neutrophil isolation protocol. J Vis Exp. 2008;23(17):745.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8.
Bertoni APS, de Campos RP, Tsao M, Braganhol E, Furlanetto TW, Wink MR. Extracellular ATP is differentially metabolized on papillary thyroid carcinoma cells surface in comparison to normal cells. Cancer Microenviron. 2018;11(1):61–70.
Chan KM, Delfert D, Junger KD. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem. 1986;157(2):375–80. https://doi.org/10.1016/0003-2697(86)90640-8.
Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:218–41.
G. Giusti BG (1984) Colorimetric Method, in: H.U. Bergmeyer (Ed.), Methods of enzymatic analysis.
Di Virgilio F, Vuerich M. Purinergic signaling in the immune system. Auton Neurosci. 2015;191:117–23.
Sumi Y, Woehrle T, Chen Y, et al. Plasma ATP is required for neutrophil activation in a mouse sepsis model. Shock. 2014;42(2):142–7.
Saito T, Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS ONE. 2015;10(3): e0118432.
Huang Z, Xie N, Illes P, et al. From purines to purinergic signalling: molecular functions and human diseases. Signal Transduct Target Ther. 2021;6(1):162.
Csóka B, Németh ZH, Rosenberger P, et al. (2010) A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185(1):542–50. https://doi.org/10.4049/jimmunol.0901295.
Haskó G, Csóka B, Koscsó B, et al. Ecto-5’-nucleotidase (CD73) decreases mortality and organ injury in sepsis. J Immunol. 2011;187(8):4256–67. https://doi.org/10.4049/jimmunol.1003379.
Németh ZH, Csóka B, Wilmanski J, et al. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176(9):5616–26. https://doi.org/10.4049/jimmunol.176.9.5616.
Csóka B, Németh ZH, Törő G, et al. Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J. 2015;9(9):3626–37. https://doi.org/10.1096/fj.15-272450.
Csóka B, Németh ZH, Szabó I, et al. Macrophage P2X4 receptors augment bacterial killing and protect against sepsis. JCI Insight. 2018;3(11):e99431. https://doi.org/10.1172/jci.insight.99431.
Martínez-García JJ, Martínez-Banaclocha H, Angosto-Bazarra D, et al. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nat Commun. 2019;10(1):2711. https://doi.org/10.1038/s41467-019-10626-x.
Pickkers P, Heemskerk S, Schouten J, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care. 2012;16(1):R14.
Li X, Kondo Y, Bao Y, et al. Systemic Adenosine Triphosphate Impairs Neutrophil Chemotaxis and Host Defense in Sepsis. Crit Care Med. 2017;45(1):e97–104.
Csoka B, Nemeth ZH, Toro G, et al. CD39 improves survival in microbial sepsis by attenuating systemic inflammation. FASEB J. 2015;29(1):25–36.
Cohen ES, Law WR, Easington CR, et al. Adenosine deaminase inhibition attenuates microvascular dysfunction and improves survival in sepsis. Am J Respir Crit Care Med. 2002;166(1):16–20.
Ramakers BP, Wever KE, Kox M, et al. How systemic inflammation modulates adenosine metabolism and adenosine receptor expression in humans in vivo. Crit Care Med. 2012;40(9):2609–16.
Nascimento DC, Viacava PR, Ferreira RG, et al. Sepsis expands a CD39(+) plasmablast population that promotes immunosuppression via adenosine-mediated inhibition of macrophage antimicrobial activity. Immunity. 2021;54(9):2024–41.
Baek SD, Kang JY, Yu H, et al. Change in alkaline phosphatase activity associated with intensive care unit and hospital length of stay in patients with septic acute kidney injury on continuous renal replacement therapy. BMC Nephrol. 2018;19(1):243.
Acknowledgements
We would like to thank all participants and their families for their participation in the current study.
Funding
This study was supported by the Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – Processo 315522/2021–6); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Código de Processamento 001). Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS – Processo 22/2551–0000388-5). P.O. de Souza, A.P.S. Bertoni, J.H. Azambuja, M. Dal Prá, L.L.P. da Cruz, and N.E. Gelsleichter were recipients of UFCSPA, CAPES or CNPq scholarships. This work was supported by the National Institutes of Health Grants R01GM06618916, R01DK11379004 and R01HL158519 awarded to GH.
Author information
Authors and Affiliations
Contributions
ROL, MRW, RMS, GH, and EB contributed to the study conception and design. Material preparation, data collection and analysis were performed by ROL, POS, FdS, KRM, APSB, MSS, JHA, MDP, LLPC, NEG, KB. The first draft of the manuscript was written by ROL, POS and CBH. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Consent for publication
All authors agree with the content of the manuscript and approved the submission.
Ethics approval and consent to participate
The current study was approved by the Research Ethics Committee (registration numbers 49959315.5.0000.5530 and 49959315.5.3001.5345). Every subject or proxy provided written informed consent before inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10238_2023_1045_MOESM1_ESM.tif
Supplementary Figure 1: In silico gene expression of ADORA2A, P2RX7 and P2RY2 receptors and NT5E, ENTPD1 and ADA enzymes in septic samples (red) compared to healthy samples (blue) in GSE137310 (A), and GSE54512 (B) datasets. (TIF 282 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leite, R.O., de Souza, P.O., Haas, C.B. et al. ATPergic signaling disruption in human sepsis as a potential source of biomarkers for clinical use. Clin Exp Med 23, 3651–3662 (2023). https://doi.org/10.1007/s10238-023-01045-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01045-w