Abstract—
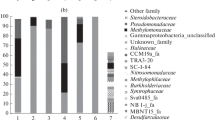
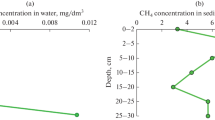
Eutrophication of lakes results in the intensification of anaerobic processes, including methanogenesis, and therefore in enhanced emission of methane. A littoral area with its variable oxygen regime is the first to react to eutrophication. The diversity of microbial communities in littoral areas is insufficiently studied, and little data are available concerning the methane cycle microorganisms. In this work, the methanogenesis and methane oxidation were investigated in the littoral site of a freshwater temperate Lake Senezh (Russia). A combination of analytical, microbiological and molecular techniques was used, including physicochemical analyses, high-throughput sequencing, potential activity measurements, and cultivation on selective media. The littoral site was found to be an extremely labile ecological niche, which harbors a diverse community containing aerobic, facultative anaerobic and anaerobic microorganisms, both autotrophs and heterotrophs, which may perform all reactions of the N, S, and CH4 cycles. Methane formation was carried out via hydrogenotrophic, acetoclastic, methylotrophic, and methyl-reducing pathways. Among methanotrophs, type I organisms predominated; type II, nitrate- and nitrite-dependent methanotrophs were also revealed. Comparison of the average rates of methanogenesis and aerobic methane oxidation suggests that all methane, which may potentially be formed in the littoral site of the lake, could simultaneously be oxidized.




Similar content being viewed by others
REFERENCES
Anderson, N.J., Bennion, H., and Lotter, A.F., Lake eutrophication and its implications for organic carbon sequestration in Europe, Glob. Chang. Biol., 2014, vol. 20, pp. 2741–2751.
Bechtel, A. and Schubert, C.J., A biogeochemical study of sediments from the eutrophic Lake Lugano and the oligotrophic Lake Brienz, Switzerland, Org. Geochem., 2009, vol. 40, pp. 1100–1114.
Billard, E., Domaizon, I., Tissot, N., Arnaud, F., and Lyauteyet, E., Multi-scale phylogenetic heterogeneity of archaea, bacteria, methanogens and methanotrophs in lake sediments, Hydrobiologia, 2015, vol. 751, pp. 159–173.
Callahan, B.J., McMurdie, P.J., Rosen, M.J., Han, A.W., Johnson, A.J.A., and Holmes, S.P., DADA2: high-resolution sample inference from Illumina amplicon data, Nat. Methods., 2016, vol. 13, pp. 581–583.
Castelle, C.J., Wrighton, K.C., Thomas, B.C., Hug, L.A., Brown, C.T., Wilkins, M.J., Frischkorn, K.R., Tringe, S.G., Singh, A., Markillie, L.M., Taylor, R.C., Williams, K.H., and Banfield, J.F., Genomic expansion of domain archaea highlights roles for organisms from new phyla in anaerobic carbon cycling, Curr. Biol., 2015, vol. 25, pp. 690–701.
Castelle, C.J., Brown, C.T., Anantharaman, K., Probst, A.J., Huang, R.H., and Banfield, J.F., Biosynthetic capacity, metabolic variety and unusual biology in the CPR and DPANN radiations, Nat. Rev. Microbiol., 2018, vol. 16, pp. 629–645.
Collins, D.A., Akberdin, I.R., and Kalyuzhnaya, M.G., Methylobacter, in Bergey’s Manual of Systematics of Archaea and Bacteria, Whitman, W.B., Rainey, F.A., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B.P., and Dedysh, S.N., Eds., New York: Wiley, 2017, pp. 1−12. https://doi.org/10.1002/9781118960608.gbm01179.pub2
Cozannet, M., Borrel, G., Roussel, E., Moalic, Y., Allioux, M., Sanvoisin, A., Toffin, L., and Alain, K., New insights into the ecology and physiology of Methanomassiliicoccales from terrestrial and aquatic environments, Microorganisms, 2021, vol.9, p. 30.
Crevecoeur, S., Vincent, W.F., Comte, J., Matveev, A., and Lovejoy, C., Diversity and potential activity of methanotrophs in high methane-emitting permafrost thaw ponds, PLoS One, 2017, vol. 12, p. e0188223.
Dedysh, S.N., Kulichevskaya, I.S., Huber, K.J., and Overmann, J., Defining the taxonomic status of described subdivision 3 Acidobacteria: proposal of Bryobacteraceae fam. nov., Int. J. Syst. Evol. Microbiol., 2017, vol. 67, pp. 498−501.
Diaz, R.J. and Rosenberg, R., Marine benthic hypoxia: a review of its ecological effects and the behavioural response of benthic macrofauna, in Oceanography and Marine Biology: An Annual Review, Ansell, A.D., Gibson, R.N., Barnes, M., Eds., London: UCL, 1995, pp. 245−303.
Diaz, R.J. and Rosenberg, R., Spreading dead zones and consequences for marine ecosystems, Science, 2008, vol. 321, pp. 926−929.
Dinasquet, J., Ortega-Retuerta, E., Lovejoy, C., and Obernosterer, I., Editorial: microbiology of the rapidly changing polar environments, Front. Mar. Sci., 2018, vol. 5, p. 154.
Dridi, B., Fardeau, M.-L., Ollivier, B., Raoult, D., and Drancourt, M., Methanomassiliicoccus luminyensis gen. nov., sp. nov., a methanogenic archaeon isolated from human faeces, Int. J. Syst. Evol. Microbiol., 2012, vol. 62, pp. 1902–1907.
Eisenberg, T., Glaeser, S.P., Nicklas, W., Mauder, N., Contzen, M., Aledelbi, K., and Kämpfer, P., Streptobacillus felis sp. nov., isolated from a cat with pneumonia, and emended descriptions of the genus Streptobacillus and of Streptobacillus moniliformis, Int. J. Syst. Evol. Microbiol., 2015a, vol. 65, pp. 2172−2178.
Eisenberg, T., Glaeser, S.P., Ewers, C., Semmler, T., Nicklas, W., Rau, J., Mauder, N., Hofmann, N., Imaoka, K., Kimura, M., and Kämpfer, P., Streptobacillus notomytis sp. nov., isolated from a spinifex hopping mouse (Notomys alexis Thomas, 1922), and emended description of Streptobacillus Levaditi et al. 1925, Eisenberg et al. 2015 emend., Int. J. Syst. Evol. Microbiol., 2015b, vol. 65, pp. 4823−4829.
Fan, X. and Xing, P., Differences in the composition of archaeal communities in sediments from contrasting zones of Lake Taihu, Front. Microbiol., 2016, vol. 7, p. 1510.
Feldewert, C., Lang, K., and Brune, A., The hydrogen threshold of obligately methyl-reducing methanogens, FEMS Microbiol. Let., 2020, vol. 367, p. fnaa137.
Fuchs, A., Lyautey, E., Montuelle, B., and Casper P., Effects of increasing temperatures on methane concentrations and methanogenesis during experimental incubation of sediments from oligotrophic and mesotrophic lakes, J. Geophys. Res. Biogeosci., 2016, vol. 121, pp. 1394–1406.
Galchenko, V.F., Methanotrophnye bacterii (Methanotrophic Bacteria), Moscow: GEOS, 2008.
Graf, J.S., Mayr, M.J., Marchant, H.K., Tienken, D., Hach, P.F., Brand, A., Schubert, C.J., Kuypers, M.M.M., and Milucka, J., Bloom of a denitrifying methanotroph, “Candidatus Methylomirabilis limnetica,” in a deep stratified lake, Environ. Microbiol., 2018, vol. 20, pp. 2598–2614.
Han, X., Schubert, C.J., Fiskal, A., Dubois, N., and Lever, M.A., Eutrophication as a driver of microbial community structure in lake sediments, Environ Microbiol., 2020, vol. 22, pp. 3446−3462.
He, R., Wooller, M.J., Pohlman, J.W., Quensen, J., Tiedje, J.M., and Leigh, M.B., Shifts in identity and activity of methanotrophs in arctic lake sediments in response to temperature changes, Appl. Environ. Microbiol., 2012, vol. 78, pp. 4715–4723.
Huber, H. and Stetter, K.O., Thermoplasmatales, in The Prokaryotes, Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., and Stackebrandt, E., Eds., New York: Springer, 2006, pp. 101–112.
Hugerth, L.W., Wefer, H.A., Lundin, S., Jakobsson, H.E., Lindberg, M., Rodin, S., Engstrand, L., and Andersson, A.F., DegePrime, a program for degenerate primer design for broad-taxonomic-range PCR in microbial ecology studies, Appl. Environ. Microbiol., 2014, vol. 80, pp. 5116–5123.
Imachi, H., Nobu, M.K., Nakahara, N., Morono, Y., Ogawara, M., Takaki, Y., Takano, Y., Uematsu, K., Ikuta, T., Ito, M., Matsui, Y., Miyazaki, M., Murata, K., Saito, Y., Sakai, S., et al., Isolation of an archaeon at the prokaryote-eukaryote interface, Nature, 2020, vol. 577, pp. 519−525.
Jeske, O., Jogler, M., Petersen, J., Sikorski, J., and Jogler, C., From genome mining to phenotypic microarrays: Planctomycetes as source for novel bioactive molecules, Antonie van Leeuwenhoek, 2013, vol. 104, pp. 551−567.
Jonkers, H.M., van der Maarel, M.J.E.C., van Gemerden, H., and Hansen, T.A., Dimethylsulfoxide reduction by marine sulfate-reducing bacteria, FEMS Microbiol. Lett., 1996, vol. 136, pp. 283−287.
Kadnikov, V.V., Savvichev, A.S., Mardanov, A.V., Beletsky, A.V., Merkel, A.Y., Ravin, N.V., and Pimenov, N.V., Microbial communities involved in the methane cycle in the near-bottom water layer and sediments of the meromictic subarctic Lake Svetloe, Antonie van Leeuwenhoek, 2019, vol. 112, pp. 1801–1814.
Kallistova, A.Yu., Merkel, A.Yu., Tarnovetskii, I.Yu., and Pimenov, N.V., Methane formation and oxidation by prokaryotes, Microbiology (Moscow), 2017, vol. 86, pp. 671−691.
Kallistova, A., Kadnikov, V., Rusanov, I., Kok-ryatskaya, N., Beletsky, A., Mardanov, A., Savvichev, A., Ravin, N., and Pimenov, N., Microbial communities involved in aerobic and anaerobic methane cycling in a meromictic ferruginous subarctic lake, Aquat. Microb. Ecol., 2018, vol. 82, pp. 1−18.
Kallistova, A.Yu., Savvichev, A.S., Rusanov, I.I., and Pimenov, N.V., Thermokarst lakes, ecosystems with intense microbial processes of the methane cycle, Microbiology (Moscow), 2019, vol. 88, pp. 649−661.
Kallistova, A., Merkel, A., Kanapatskiy, T., Bol-tyanskaya, Y., Tarnovetskii, I., Perevalova, A., Kevbrin, V., Samylina, O., and Pimenov, N., Methanogenesis in the Lake Elton saline aquatic system, Extremophiles, 2020, vol. 24, pp. 657–672.
Kallistova, A.Yu., Kadnikov, V.V., Savvichev, A.S., Rusanov, I.I., Dvornikov, Yu.A., Leibman, M.O., Khomutov, A.V., Ravin, N.V., and Pimenov, N.V., Comparative study of methanogenic pathways in the sediments of thermokarst and polygenetic Yamal lakes, Microbiology (Moscow), 2021, vol. 90, pp. 261–267.
Kantor, R.S., van Zyl, A.W., van Hille, R.P., Thomas, B.C., Harrison, S.T., and Banfield, J.F., Bioreactor microbial ecosystems for thiocyanate and cyanide degradation unraveled with genome-resolved metagenomics, Environ. Microbiol., 2015, vol. 17, pp. 4929−4941.
Kodama, Y. and Watanabe, K., Sulfuricurvum kujiense gen. nov., sp. nov., a facultatively anaerobic, chemolithoautotrophic, sulfur-oxidizing bacterium isolated from an underground crude-oil storage cavity, Int. J. Syst. Evol. Microbiol., 2004, vol. 54, pp. 2297−2300.
Kojima, H. and Fukui, M., Sulfuricella denitrificans gen. nov., sp. nov., a sulfur-oxidizing autotroph isolated from a freshwater lake, Int. J. Syst. Evol. Microbiol., 2010, vol. 60, pp. 2862−2866.
Kojima, H. and Fukui, M., Sulfuritalea hydrogenivorans gen. nov., sp. nov., a facultative autotroph isolated from a freshwater lake, Int. J. Syst. Evol. Microbiol., 2011, vol. 61, pp. 1651−1655.
Kojima, H., and Fukui, M., Sulfurisoma sediminicola gen. nov., sp. nov., a facultative autotroph isolated from a freshwater lake, Int. J. Syst. Evol. Microbiol., 2014, vol. 64, pp. 1587−1592.
Konstantinov, A.S., Obshchaya gidrobiologiya (General Hydrobiology), Moscow: Vysshaya shkola, 1986, 4th ed.
Kulichevskaya, I.S., Suzina, N.E., Rijpstra, W.I.C., Damsté, J.S.S., and Dedysh, S.N., Paludibaculum fermentans gen. nov., sp. nov., a facultative anaerobe capable of dissimilatory iron reduction from subdivision 3 of the Acidobacteria, Int. J. Syst. Evol. Microbiol., 2014, vol. 64, pp. 2857−2864.
Li, Q.M., Zhou, Y.L., Wei, Z.F., and Wang, Y., Phylogenomic insights into distribution and adaptation of Bdellovibrionota in marine waters, Microorganisms, 2021, vol. 9, p. 757.
Liu, X., Li, M., Castelle, C.J., Probst, A.J., Zhou, Z., Pan, J., Liu, Y., Banfield, J.F., and Gu, J.D., Insights into the ecology, evolution, and metabolism of the widespread Woesearchaeotal lineages, Microbiome, 2018, vol. 6, p. 102.
Lyautey, E., Billard, E., Tissot, N., Jacquet, S., and Domaizon, I., Seasonal dynamics of abundance, structure, and diversity of methanogens and methanotrophs in lake sediments, Microb. Ecol., 2021, vol. 82, pp. 559−571.
Martinez-Cruz, K., Leewis, M.C., Herriott, I.C., Sepulveda-Jauregui, A., Anthony, K.W., Thalasso, F., and Leigh, M.B., Anaerobic oxidation of methane by aerobic methanotrophs in sub-Arctic lake sediments, Sci. Total Environ., 2017, vols. 607–608, pp. 23–31.
Martynov, A.A., Spravochnik rybolova-sportsmena Podmoscow’ya (Sports Angler Handbook of the Moscow Region), Moscow: Moskovskaya Pravda, 1988, 2nd ed.
Martin, G., Rissanen, A.J., Garcia, S.L., Mehrshad, M., Buck, M., and Peura, S., Candidatus Methylumidiphilus drives peaks in methanotrophic relative abundance in stratified lakes and ponds across northern landscapes, Front. Microbiol., 2021, vol. 12, p. 669937.
Mayr, M.J., Zimmermann, M., Guggenheim, C., Brand, A., and Bürgmann, H., Niche partitioning of methane-oxidizing bacteria along the oxygen-methane counter gradient of stratified lakes, ISME J., 2020, vol. 14, pp. 274−287.
McAuliffe, C., Gas chromatographic determination of solutes by multiple phase equilibrium, Chem. Technol., 1971, vol. 1, pp. 46–51.
McMurdie, P.J. and Holmes, S., Phyloseq: a bioconductor package for handling and analysis of high-throughput phylogenetic sequence data, Pac. Symp. Biocomput., 2012, pp. 235–246.
Merkel, A.Yu., Tarnovetskii, I.Yu., Podosokorskaya, O.A., and Toshchakov, S.V., Analysis of 16S rRNA primer systems for profiling of thermophilic microbial communities, Microbiology (Moscow), 2019, vol. 88, pp. 671–680.
Moschos, S., Piperagkas, O., and Karayanni, H.A., Vertically and temporally diverse bacterial community in a shallow lake-water sediment site of a eutrophic lake, Inland Waters, 2021, vol. 11, pp. 141−153.
Nakagawa, Y. and Yamasato, K., Emendation of the genus Cytophaga and transfer of Cytophaga agarovorans and Cytophaga salmonicolor to Marinilabilia gen. nov.: phylogenetic analysis of the Flavobacterium-Cytophaga complex. Int. J. Syst. Bacteriol. 1996, vol. 46, pp. 599−603.
Nevin, K.P., Holmes, D.E., Woodard, T.L., Hinlein, E.S., Ostendorf, D.W., and Lovley, D.R., Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., two novel Fe(III)-reducing subsurface isolates, Int. J. Syst. Evol. Microbiol., 2005, vol. 55, pp. 1667−1674.
Ortiz-Alvarez, R. and Casamayor, E.O., High occurrence of Pacearchaeota and Woesearchaeota (Archaea superphylum DPANN) in the surface waters of oligotrophic high-altitude lakes, Environ. Microbiol. Rep., 2016, vol. 8, pp. 210–217.
Pfennig, N., Anreicherungskulturen für rote und grüne Schwefelbakterien, Zbl. Bakt. Hyg., I. Abt. Orig., 1965, Su-ppl. 1, vols. 179−189, pp. 503−504.
Pfennig, N. and Lippert, K.D., Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien, Archiv. Mikrobiol., 1966, vol. 55, pp. 245–256.
Pimenov, N.V., Kallistova, A.Yu., Rusanov, I.I., Yusupov, S.K., Montonen, L., Jurgens, G., Münster, U., Nozhevnikova, A.N., and Ivanov, M.V., Methane formation and oxidation in the meromictic oligotrophic Lake Gek-Gel (Azerbaijan), Microbiology (Moscow), 2010, vol. 79, pp. 247−252.
Preston, D.L., Caine, N., McKnight, D.M., Williams, M.W., Hell, K., Miller, M.P., Hart, S.J., and Johnson, P.T.J., Climate regulates alpine lake ice cover phenology and aquatic ecosystem structure, Geophys. Res. Lett., 2016, vol. 43, pp. 5353–5360.
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., Peplies, J., and Glöckner, F.O., The SILVA ribosomal RNA gene database project: improved data processing and web-based tools, Nucleic Acids Res., 2013, vol. 41, pp. D590−D596.
Reis, P.C.J., Thottathil, S.D., Ruiz-González, C., and Prairie, Y.T., Niche separation within aerobic methanotrophic bacteria across lakes and its link to methane oxidation rates, Environ. Microbiol., 2020, vol. 22, pp. 738−751.
Reis, P.C.J., Thottathil, S.D., and Prairie, Y.T., The role of methanotrophy in the microbial carbon metabolism of temperate lakes, Nat. Commun., 2022, vol. 13, p. 43.
Ren, Y., Yu, M., Low, W.Y., Ruhlman, T.A., Hajrah, N.H., El Omri, A., Alghamdi, M.K., Sabir, M.J., Alhebshi, A.M., Kamli, M.R., Sabir, J.S.M., Theriot, E.C., Jansen, R.K., and Rather, I.A., Nucleotide substitution rates of diatom plastid encoded protein genes are positively correlated with genome architecture, Sci. Rep., 2020, vol. 10, p. 14358.
Renaud, G., Stenzel, U., Maricic, T., Wiebe, V., and Kelso, J., deML: robust demultiplexing of Illumina sequences using a likelihood-based approach, Bioinformatics, 2015, vol. 31, pp. 770–772.
Rissanen, A.J., Saarenheimo, J., Tiirola, M., Peura, S., Aalto, S.L., Karvinen, A., and Nykänen, H., Gammaproteobacterial methanotrophs dominate methanotrophy in aerobic and anaerobic layers of boreal lake waters, Aquat. Microb. Ecol., 2018, vol. 81, pp. 257–276.
Rosentreter, J.A., Borges, A.V., Deemer, B.R., Holger-son, M.A., Liu, S., Song, C., Melack, J., Raymond, P.A., Duarte, C.M., Allen, G.H., Olefeldt, D., Poulter, B., Battin, T.I., and Eyre, B.D., Half of global methane emissions come from highly variable aquatic ecosystem sources, Nat. Geosci., 2021, vol. 14, pp. 225–230.
Sanford, R.A., Cole, J.R., and Tiedje, J.M., Characterization and description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an aryl-halorespiring facultative anaerobic myxobacterium, Appl. Environ. Microbiol., 2002, vol. 68, pp. 893−900.
Savvichev, A.S., Kadnikov, V.V., Rusanov, I.I., Belet-sky, A.V., Krasnova, E.D., Voronov, D.A., Kallistova, A.Yu., Veslopolova, E.F., Zakharova, E.E., Kokryatskaya, N.M., Losyuk, G.N., Demidenko, N.A., Belyaev, N.A., Sigalevich, P.A., Mardanov, A.V., et al., Microbial processes and microbial communities in the water column of the Polar meromictic Lake Bol’shie Khruslomeny at the White sea coast, Front. Microbiol., 2020, vol. 11, p. 1945.
Savvichev, A., Rusanov, I., Dvornikov, Y., Kadnikov, V., Kallistova, A., Veslopolova, E., Chetverova, A., Leibman, M., Sigalevich, P., Pimenov, N., Ravin, N., and Khomutov, A., The water column of the Yamal tundra lakes as a microbial filter preventing methane emission, Biogeosciences, 2021, vol. 18, pp. 2791–2807.
Sleat, R., Mah, R.A., and Robinson, R., Acetoanaerobium noterae gen. nov., sp. nov.: an anaerobic bacterium that forms acetate from H2 and CO2, Int. J. Syst. Bacteriol., 1985, vol. 35, pp. 10−15.
Spring, S., Kampfer, P., Ludwig, W., and Schleifer, K.-H., Polyphasic characterization of the genus Leptothrix: new descriptions of Leptothrix mobilis sp. nov. and Leptothrix discophora sp. nov. nom. rev. and emended description of Leptothrix cholodnii emend., System. Appl. Microbiol., 1996, vol. 19, pp. 634−643.
Storesund, J.E., Lanzèn, A., Nordmann, E.-L., Armo, H.R., Lage, O.M., and Øvreås, L., Planctomycetes as a vital constituent of the microbial communities inhabiting different layers of the meromictic Lake Sælenvannet (Norway), Microorganisms, 2020, vol. 8, p. 1150.
Su, G., Zopfi, J., Niemann, H., and Lehmann, M.F., Multiple groups of methanotrophic bacteria mediate methane oxidation in anoxic lake sediments, Front. Microbiol., 2022, vol. 13, p. 864630.
Suominen, S., Dombrowski, N., Sinninghe Damsté, J.S., and Villanueva, L., A diverse uncultivated microbial community is responsible for organic matter degradation in the Black Sea sulfidic zone, Environ. Microbiol., 2021, vol. 23, pp. 2709−2728.
Tardy, V., Etienne, D., Masclaux, H., Essert, V., Millet, L., Verneaux, V., and Lyautey, E., Spatial distribution of sediment archaeal and bacterial communities relates to the source of organic matter and hypoxia—a biogeographical study on Lake Remoray (France), FEMS Microbiol. Ecol., 2021, vol. 97, p. fiab126.
Toshchakov, S.V., Izotova, A.O., Vinogradova, E.N., Kachmazov, G.S., Tuaeva, A.Y., Abaev, V.T., Evteeva, M.A., Gunitseva, N.M., Korzhenkov, A.A., Elcheninov, A.G., Patrushev, M.V., and Kublanov, I.V., Culture-independent survey of thermophilic microbial communities of the North Caucasus, Biology, 2021, vol. 10, p. 1352.
Trojan, D., Schreiber, L., Bjerg, J.T., Bøggild, A., Yang, T., Kjeldsen, K.U., and Schramm, A.A., Taxonomic framework for cable bacteria and proposal of the candidate genera Electrothrix and Electronema, Syst. Appl. Microbiol., 2016, vol. 39, pp. 297−306.
van Grinsven, S., Meier, D.V., Michel, A., Han, X., Schubert, C.J., and Lever, M.A., Redox zone and trophic state as drivers of methane-oxidizing bacterial abundance and community structure in lake sediments, Front. Environ. Sci., 2022, vol. 10, p. 857358.
Wang, J., Wei, Z.P., Chu, Y.X., Tian, G., and He, R., Eu-trophic levels and algae growth increase emissions of methane and volatile sulfur compounds from lakes, Environ. Pollut., 2022, vol. 306, p. 119435.
Wolin, E.A., Wolin, M.J., and Wolfe, R.S., Formation of methane by bacterial extracts, J. Biol. Chem., 1963, vol. 238, pp. 2882−2886.
Yamada, T., Sekiguchi, Y., Hanada, S., Imachi, H., Ohashi, A., Harada, H., and Kamagata, Y., Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi, Int. J. Syst. Evol. Microbiol., 2006, vol. 56, pp. 1331−1340.
Yang, Y., Chen, J., Tong, T., Li, B., He, T., Liu, Y., and Xie, S., Eutrophication influences methanotrophic activity, abundance and community structure in freshwater lakes, Sci. Total Environ., 2019, vol. 662, pp. 863−872.
ACKNOWLEDGMENTS
The authors are thankful to LLC “MGULAB” (https://www.msulab.ru/) for the chemical analysis of water samples and to Prof. N.V. Ravin (Research Centre of Biotechnology RAS) for critical reading of the manuscript and useful recommendations.
Funding
This research was partially funded by Russian Science Foundation, grant no. 22-14-00038 (activity measurements, enrichments isolation, sequencing of native samples and methanogenic enrichments). Sequencing of the methanotrophic enrichment and bioinformatic analyses were funded by Russian Foundation for Basic Research according to the research project no. 20-04-60190. Field work was supported by State Assignment for the Laboratory of relict microbial communities, Research Centre of Biotechnology RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Rights and permissions
About this article
Cite this article
Kallistova, A.Y., Koval, D.D., Kadnikov, V.V. et al. Methane Cycle in a Littoral Site of a Temperate Freshwater Lake. Microbiology 92, 153–170 (2023). https://doi.org/10.1134/S0026261722602901
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722602901