Abstract
The Yessentukskoye deposit of Caucasian mineral waters contains balneologically valuable drinking mineral water, which is extracted from the Upper Cretaceous 1 km subsurface aquifer and is almost unexplored by microbiologists. We have sampled this water via continuously operating production wells, characterized the phylogenetic diversity of its microbial community, and obtained enrichments of thermophilic iron reducers from the source aquifer. From the enrichments, a novel anaerobic thermophilic bacterium, reducing Fe(III) in the mineral ferrihydrite with acetate as the electron donor, was isolated into a pure culture. The novel isolate, designated as strain Es71-Z0220T belonging to Deferribacterales order, is thermophilic, neutrophilic, halotolerant, motile vibrio. It utilizes synthesized ferrihydrite, fumarate, nitrate or elemental sulfur as the electron acceptors with organic acids as the electron donors. The strain is incapable of soluble Fe(III) complexes reduction and fermentative growth. The draft genome assembly of strain Es71-Z0220T resulted in 65 contigs with a total size of ca. 2.3 Mb. On the basis of whole-genome phylogenetic reconstruction and physiological characterization, the novel isolate was considered to represent a novel family, genus and species for which the name Deferrivibrio essentukiensis gen. nov., sp. nov. is proposed. Genome analysis revealed key determinants of anaerobic respiration and carbon substrate utilization pathways in the organism with peculiarities related to putative Fe(III)-reducing electron transfer chain. Considering the revealed metabolic features of Deferrivibrio essentukiensis, the involvement of the organism in its subsurface environment in biogeochemical by carbon cycling by coupling the organic matter oxidation with Fe(III) minerals reduction is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Drinking mineral waters represent one of the most significant natural resources. Officially, only microbiologically unaltered waters are classified as the mineral ones. Natural springs or drilled subsurface sources of mineral waters are the subjects of thorough protection intended to guarantee their original microbiological purity and chemical composition upon exploitation (Loy et al., 2005). This is not surprising, considering that significant influence of microbial metabolic activity on the genesis of mineral waters has been assumed quite long ago (Leclerc et al., 2005). However, the phylogenetic and metabolic diversity of microbial communities and individual microorganisms inhabiting subsurface aquifers remain poorly characterized. In this regard, there should be mentioned recent works describing the community profiles of several European bottled mineral waters and their natural sources (Lesaulnier et al., 2017; Krauze et al., 2017; Sala-Comorera et al., 2020). These studies revealed the predominance of Gammaproteobacteria in the majority of analyzed communities and significant differences in their phylogenetic profiles at the level of orders and families. At the same time, the involvement of microbiota of mineral waters in the formation of their composition was left almost unexplored. Microbiological research on this issue could be an essential measure for the assessment of mineral water resources. When applied to subsurface mineral water deposits, this measure provides microbiologists with a unique opportunity to study the autochthonous microbiota of subsurface aquifers, inaccessible for direct sampling, and estimate its geochemical activity and ecological role.
The subject of our study was the Yessentukskoye mineral water deposit (YMWD) which is located in the Kislovodsk-Kumagorsky tectonic fracturing zone (pre-Caucasus) and has complex hydrogeological settings due to the monoclinal geological structure. The main hydrogeochemical feature of YMWD is the gas-hydrogeochemical anomaly (inversion) of mineral waters. Its formation was facilitated by a peculiar paleohydrogeodynamic regime in the horizons of different ages and by the processes of the Alpine cycle of geological evolution of the entire Caucasian region, which led to the formation of a complex system of multidirectional sublatitudinal and submeridional linear and circular fault zones in the sedimentary rocks. Geological complexity of the region is considered to condition the great diversity of mineral waters at YMWD with mineralization ranging from practically fresh (0.5–0.9 g L–1) to highly mineralized waters (10.0–14.0 g L–1), temperatures ranging from 10 to ~70°С, and variability of predominant anions (carbonate, bicarbonate, chloride or sulfate ions) (Abramov and Vavichkin, 2021; Lavrushin et al., 2020; Filimonova et al., 2020). The system of fault zones in the region serves as the channel for juvenile gases, primarily СО2 and Н2 (Filimonova et al., 2020), which could supply the chemotrophic part of a microbial community with carbon and energy sources (e.g., in the processes of methano- or acetogenesis). Combination of these sources with inorganic electron acceptors, first of all, with Fe(III) minerals and sulfur compounds which are widely available in water-bearing rocks, opens the potential for a great diversity of metabolic pathways to sustain microbial life in the aquifers of YMWD. Microbial communities of these subsurface ecosystems have attracted the researchers’ attention for several decades but mainly cultivated sulfate reducers or opportunistic pathogens capable of dispersed organic matter degradation were in the focus of previous microbiological researches in the region (refer to Potapov et al., 2014, 2017, and references therein).
In 2018, a large program on the reassessment of subsurface mineral water resources was started on YMWD. Within the program, many exploitation wells operated at the highest productivity over a long period, which allowed sampling of the subsurface mineral water extracted from the aquifers of up to 1 km depth directly through the wellheads, avoiding bias related to water stagnation in the wellbore. As a part of this program, water sampling from 2 wells for microbiological studies was performed. Both wells drilled the same Upper Cretaceous aquifer from which one of the most balneologically valuable types of YMWD mineral water (type “Yessentuki no. 4”) is extracted.
The aim of our work was the description of physiological and genomic features of dissimilatory iron-reducing bacterium isolated from the sampled subsurface mineral waters of YMWD and representing novel species, genus, and family within the order Deferribacterales of Deferribacterota phylum.
MATERIALS AND METHODS
Sampling and enrichments. We have sampled mineral waters from the well 71 in September 2020 and from the well 71N in July 2021. Both wells (E 42°56′30′′ N 44°11′20′′) are located 14 m from each other and have depths of 999 m with open boreholes in the interval of 676.0–998.9 m. Mineral subsurface drinking water is extracted from an Upper Cretaceous (Cenomanian-Maastrichtian) aquifer (K2s-m), spreading along the area of YMWD. The total thickness of the aquifer varies from 300 to 350 m, its sediments contain limestones, marl, bundles of sandstone, and are characterized by complicated hydrodynamic conditions. The well 71 is utilized for industrial extraction of mineral water Yessentuki no. 4, while the well 71N is used as a backup of the well 71. Samples from both wells were used as inocula for the isolation of Fe(III)-reducing microorganisms that could thrive in sedimentary rocks of the subsurface aquifer. Samples from the main production well 71 were also used for DNA extraction and further phylogenetic profiling of the microbial community of the mineral water. To assess the phylogenetic diversity of the microbial community of the extracted mineral water, samples for subsequent DNA isolation were taken from the production wellhead 71 using FM02-1000 membrane filtration units with a volume of 1 L (Institute for Analytical Instrumentation of RAS, St. Petersburg, Russia) with 0.2 μm pore size track membrane filters (JINR, Dubna, Russia). Pre-sterilized filtration units were assembled on-site, connected to the wellhead fittings through sterile connectors and hoses, and 100 L of water per each sample were passed through the filters under natural excess pressure of the wells (4–6 atm at the wellhead). At the end of filtration, the residual water was completely pushed through the filters by the gases outgoing from the wells, the filters were soaked in buffer A (100 mM Tris-HCl, pH 8.0; 100 mM EDTA; 150 mM NaCl), transferred to the laboratory in sterile 15 mL Falcon tubes on ice and frozen at ‒20oC for storage until DNA extraction.
Sterile 17 mL Hungate anaerobic culture tubes, pre-filled with 100% CO2 gas and substrates necessary for the development of target metabolic groups of microorganisms, were used to obtain enrichment cultures. 10 mL of fresh water samples were injected into the tubes with sterile syringes, and the tubes were further incubated in the dark at 47°C—the average temperature observed during well operation in tap mode. Thus, water samples served as both the basic mineral medium and the inoculum for primary enrichments.
Community profiling by 16S rRNA gene fragments sequencing. For 16S rRNA gene fragments profiling of the water microbial community, DNA was extracted directly from frozen filters using the FastDNA Spin Kit for Soil (MP Bio, United States ) according to the manufacturer’s instructions. Preparation of V4 16S rRNA gene amplicon libraries was performed as described previously (Gohl et al., 2016), using the primer pair 515F (5'-GTGBCAGCMGCCGCGGCGGTAA-3' (Hugerth et al., 2014))—Pro-mod-805R (5'-GACTACNVGGGTMTCTAATCC-3' (Merkel et al., 2019)). Libraries were made in duplicates and sequenced on a MiSeq system (Illumina, California, United States ) using a 150-nucleotide length paired-end read cartridge. Bioinformatic analysis of 16S rRNA gene profiles was performed according to (Merkel et al., 2021). All 16S rRNA gene sequencing data were deposited in NCBI BioProject PRJNA760784.
Genome sequencing, assembly, annotation, and phylogenetic analysis. Genomic DNA of strain Es71-Z0220T was extracted with the FastDNA Spin Kit (MP Bio, United States ). The genome was sequenced on a NextSeq system (Illumina, California, United States ) using the reagent kit providing for 2 × 100 bp reading. Assembly of genomic reads by Unicycler v0.4.8 (Wick et al., 2017) resulted in 65 contigs with a total size of 2 362 387 bp (N50 value—77 140 bp). Final assembly coverage was 1207×. Automatic annotation was done by PGAP 2021-07-01 (Tatusova et al., 2016). This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JAJAFU000000000.
Refining of the automated annotations and other predictions were done manually as previously described (Toshchakov et al., 2018). Multiheme cytochromes were predicted using amino acid sequences of reported cytochromes, involved in extracellular electron transfer (EET) of Geobacter sulfurreducens (Butler et al., 2010; Aklujkar et al., 2013), Shewanella oneidensis (Coursolle et al., 2010; Schicklberger et al., 2013), “Thermincola potens” (Carlson et al., 2012; Costa et al., 2019), and Carboxydothermus ferrireducens (Gavrilov et al., 2021) as queries. Heme-binding motifs were predicted as previously described (Mardanov et al., 2015). Subcellular localization of multiheme cytochromes was predicted basing on consensus results of online prediction services TMHMM 2.0 (at CBS Prediction Servers, http://www.cbs.dtu.dk/services) and Phobius (http://phobius.sbc.su.se/). Conserved domains were predicted with HMMSCAN (https://www.ebi.ac.uk/Tools/hmmer/search/hmmscan) considering Pfam, TIGRFAM, Gene3D, Superfamily, PIRSF and TreeFam protein families databases.
For genome-based phylogenetic reconstructions, 120 bacterial single copy conservative marker genes were used as described previously (Parks et al., 2020). The trees were built using the IQ-TREE 2 program (Nguyen et al., 2015) with fast model selection via ModelFinder (Kalyaanamoorthy et al., 2017) and ultrafast approximation for phylogenetic bootstrap (Hoang et al., 2018), as well as approximate likelihood-ratio test for branches (Anisimova and Gascuel, 2006).
Physiological studies. Basal medium (A) used for pure culture isolation contained (g/L): NH4Cl, 0.33; KCl, 0.33; KH2PO4, 0.33; CaCl2·2H2O, 0.33; MgCl2·6H2O, 0.33; NaCl, 2.0; NaHCO3, 6.0; Na2S·9H2O, 0.5; 1 mL/L of trace mineral solution (Kevbrin and Zavarzin, 1992); 1 mL/L of vitamin solution (Wolin et al., 1963). Sodium acetate (20 mM) was used as the main carbon source and the electron donor, and synthesized ferrihydrite (SF) served as the target electron acceptor for Fe(III) reducing microorganisms. SF was prepared as described previously (Zavarzina et al., 2006) and added to the culture medium up to the final Fe(III) content of 50 mM before sterilization. The medium was prepared anaerobically under 100% CO2 gas (extra pure grade), dispensed by 10 mL into Hungate tubes under CO2 efflux, and autoclaved at 121°C for 20 min. The pH of the medium (A) after sterilization was 6.7. The obtained pure culture was first tested for growth with a soluble electron acceptor fumarate (20 mM). After successful tests, optimal NaCl and NaHCO3 concentrations for the growth of novel isolate were determined, and the medium was further optimized by adding 7.0 g/L sodium chloride, 6.0 g/L sodium bicarbonate, and 0.3 g/L Na2S·9H2O as a reducing agent, pH of this medium after autoclaving comprised 7.1. The optimized medium (B) was used for further physiological characterization of the isolate. To determine the spectrum of electron acceptors utilized by the isolate, crotonate (10 mM), anthraquinone disulfonate (AQDS) (10 mM), elemental sulfur (1% w/v), sulfate (20 mM), sulfite (2 mM), thiosulfate (10 mM), dimethylsulfoxide (10 mM), selenite (5 mM), nitrate (10 mM), or soluble Fe(III) forms (ferric citrate or Fe(III)-EDTA, 10 mM each) were added to the medium (B). Growth on these electron acceptors was tested with acetate as the electron donor. In the media supplemented with nitrate, SF, or soluble Fe(III) compounds, the reducing agent was omitted. For routine transfers, antibiotics resistance tests, temperature and pH optima determination, the medium (B), supplemented with sodium fumarate and reduced with Na2S·9H2O, was utilized. Incubation was performed at 47°C, unless otherwise noted. All physiological tests were performed in duplicate.
Cell morphology was characterized by phase-contrast and fluorescence microscopy using Axio Lab.A1 microscope (Zeiss, Germany). Ultrafine cell structure was visualized by JEM-100 electron microscope (JEOL, Japan) as described previously (Zavarzina et al., 2006). Cell growth was determined by direct cell counting using phase-contrast microscopy. To trace the growth of the cultures with insoluble electron acceptor (SF), subsamples were stained with acridine orange dye for DNA and further visualized using fluorescence microscopy.
To determine optimal growth conditions of the isolate, temperatures ranging from 24 to 65°C, NaCl concentrations of up to 25 g/L, NaHCO3 concentrations of up to 20 g/L, and pH ranging from 6.0 to 8.15 were tested using optimized medium (B). Cell counts were performed within 2−14 days of incubation. Optimal NaCl concentrations for growth were determined on a modified medium B in which all chlorides were replaced by equimolar concentrations of nitrates or sulfates. pH of all the used media was adjusted with 6 M HCl or 12 M NaOH solutions, alkaline media for determination of pH optimum were prepared under N2/CO2 (80 : 20, v/v) gas mixture.
Aerobic and microaerobic growth was tested using the medium (B) lacking the reducing agent, under 2, 4, 10% O2 (in CO2) or 100% air in the gas phase. Catalase activity was tested by bubble production assay with 3% (v/v) H2O2, oxidase activity was determined with 1% (w/v) tetramethyl-p-phenylenediamine (Cappuccino et al., 2002).
In addition to acetate, a set of the following carbon substrates and electron donors was tested: lactate, pyruvate, succinate, formate, malate, citrate, methanol, ethanol, n-propanol, butanol, N-acetyl-D-glucosamine, glucose, sucrose, cellobiose, microcrystalline- and carboxymethylcellulose, peptone, yeast extract, beef extract, and tryptone. Also, molecular hydrogen (100% in the gas phase) was tested as the electron donor in the absence of organic compounds. The growth was considered positive if no decrease in the cell yield was observed within three consecutive transfers on the same medium with the same substrate, and if the cell counts were three-fold greater than those in the control substrate-free experiment. All the organic substrates (peptides, carbohydrates, alcohols, and organic acids) were filter-sterilized using 0.2 µm pore size syringe filters (Millipore) and were added to a final concentration of 0.3 wt %. The utilization of non-fermentable substrates was tested with SF as the electron acceptor. The capability for anaerobic respiration was studied on the optimized medium (B) with acetate as the substrate. Sensitivity to antibiotics was tested on the same medium with fumarate, acetate, and sodium sulfide. Concentrated individual antibiotic solutions were filter-sterilized with syringe filters and injected into the tubes with sterile medium.
Acetate consumption was determined using a Crystal 5000.2 gas chromatograph (Chromatech, Russia) equipped with a TCD detector and a molecular sieve 5A column (1 m) with argon as the carrier gas. Subsamples for chromatography were obtained by centrifugation (12000 g for 3 min) of culture broth followed by adjustment of pH in the supernatant to 2.0 with extra pure grade HCOOH.
Sulfide production during the growth on sulfur compounds was determined according to Trüper and Schlegel (1964). Fe(II) production during the growth with Fe(III) compounds was monitored with ferrozine (Stookey, 1970), Fe(II) from SF and magnetite was preliminary extracted with 0.6 N HCl, AQDS reduction was monitored by the color change of the medium.
During the growth on nitrate, nitrite production was estimated with nitrite test strips (MerckQuant, Merck, Germany) and ammonium concentration was determined with indophenol as previously described (Scheiner, 1976).
For fatty acid analysis, the isolate was grown at optimal physico-chemical growth conditions. Biomass was collected in the late exponential growth phase. Cellular fatty acid profiles were determined by GC–MS (Thermo Scientific Trace GC Ultra DSQ II, HP-5MS column, EI70 eV) of methyl ester derivatives prepared from 5 mg of freeze-dried biomass treated by anhydrous HCl/MeOH, based on retention time (using Supelco standards, Merck, Germany), reference equivalent chain length values (Härtig, 2008), and mass spectra (NIST MS Search 2.0 program provided with the GC–MS setup). Cellular fatty acids content was determined as percentages of the total ion current peak area.
Isoprenoid quinones were extracted according to Collins (Collins and Jones, 1981; Collins, 1985) and analyzed using an LCQ ADVANTAGE MAX tandem-type mass spectrometer and Finnigan Mat 8430 ionization mass spectrometer.
Spectrophotometric detection of c-type cytochromes was performed basing on the rapid scanning of the UV-visible light absorbance spectrum of microbial cells or their fractions, allowing determination of the redox state of cytochromes by specific absorbance peaks, as described previously (Mardanov et al., 2015). In the current study, the assay was modified: the cytochromes were detected directly in whole-cell suspension of fumarate-grown cells, and reoxidation of dithionite-reduced suspension was tested with soluble ferric citrate (1 mM). The incubation temperature corresponded to the growth optimum (47°C) of the isolate.
RESULTS
Characteristics of the sampling site. The extracted mineral water belongs to the mineral medicinal drinking waters of the Yessentuki no. 4 type. The main physico-chemical characteristics of water from the wells 71 and 71N for the time of sampling are presented in Table 1. These characteristics allow us to characterize the extracted water as thermal, slightly alkaline, carbonaceous mineral water.
Phylogenetic profile of microbial community of the mineral water extracted from 71 wellhead. Analysis of the mineral water extracted from 71 wellhead by 16S rRNA gene profiling revealed the predominance of hydrogenotrophic methanogenic archaea of Methanothermobacter genus (37%). These archaea together with phylotypes belonging to Methanobacterium genus and Methanomicrobiaceae family represent the most abundant group of the microbial community and comprised 41% of all the 16S rRNA gene reads (Fig. S1). The second most abundant group of the microbial community was represented by phylotypes belonging to sulfate-reducing hydrogenotrophic members of Thermodesulfovibrio genus (19%). 12% of the reads were represented by unclassified phylotypes of phylum “Spirochaetota” and 9%—by completely unclassified bacterial phylotypes. A deep phylogenetic lineage of uncultured actinobacteria (Coriobacteriia class, OPB41 family-level group) was represented by 6% of all the reads. Unclassified phylotypes of Rhodocyclaceae and various uncultured Firmicutes comprised 3 and 5% of all the reads, respectively.
Among minor groups of the community, each representing less than 1% of the reads, were revealed several taxa harboring known Fe(III)-reducing microorganisms, one of which was the order Deferribacterales represented by a single sequence.
Enrichment and isolation. Active growth of iron-reducing microorganisms was observed after four days of incubation of a primary enrichment culture with mineral water of 71 well, SF, and acetate. The growth was accompanied by the transformation of brown-colored SF to a black magnetic mineral, presumably magnetite. Microscopy of enrichment subsamples, stained with acridine orange, revealed the presence of vibrio-like cells and very small rods that were associated with mineral particles, as well as planktonic coccoid cells of various diameters.
16S rRNA gene-based profiling of the primary enrichment revealed the predominance of novel representatives of the order Deferribacterales (79% relative abundance), which were fairly distantly related to the closest cultivated organism Petrothermobacter organi-vorans (93.63% 16S rRNA gene fragment identity). This phylotype was accompanied by the representatives of uncultured actinobacterial order OPB41 (3% relative abundance), spirochaetes of Rectinema cohabitans species (13%), and representatives of Caldicoprobacter genus (5%) (Fig. S2). The dominating bacterium was isolated into a pure culture by serial ten-fold dilutions. The last positive dilution (10−7) contained morphologically homogeneous motile vibrio-shaped cells. The purity of the culture designated as strain Es71-Z0220T (=VKM B-3557 = JCM 39245) was confirmed by 16S rRNA gene sequencing.
Application of the same enrichment conditions to a water sample from 71N well, taken 9 months after the strain Es71-Z0220T was isolated, led to the isolation of another novel representative of Deferribacterales order. Full-length 16S rRNA gene sequence analysis revealed this representative, designated as strain Es71N, to belong to the same species as the strain Es71-Z0220T (99.67% complete 16S rRNA gene sequence identity).
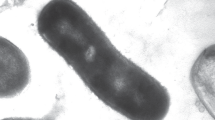
Phenotypic and chemotaxonomic characterization. Cells of strain Es71-Z0220T were vibrios of 0.4–0.5 μm width and 1–3 μm length, occurring singly, in pairs, or in long chains. The chains were observed only at suboptimal growth conditions (Fig. 1a). At the late exponential growth phase, cells formed spheroplasts and were often accumulated in dense tangles of long chains. Spores have never been observed. Cells were motile by means of one polar flagellum (Fig. 1b) and revealed a gram-negative cell wall structure (Fig. 1c).
Morphology of the cells of strain Es71-Z0220T. (a) Phase-contrast image of a culture grown with fumarate and acetate, taken at the stationary growth phase, (b) TEM image of a cell with a flagellum, (c) TEM image of an ultrathin section of a cell indicating gram-negative structure of the cell wall. Scale bars: on (a) 10 µm; on (b) and (c) 0.5 µm.
Strain Es71-Z0220T was an obligate anaerobe. No growth was observed in aerobic medium or at 2 % or higher O2 content in the gas phase. Moreover, the cells rapidly lysed on air and their number in an opened Hungate tube halved in 45 min. However, the strain appeared to be catalase and oxidase positive. It was moderate thermophile growing at the temperature range of 30 to 54 °C, with an optimum at 47 °C. No growth occurred at 24 and 60°C. The pH range for growth at 47°C comprised 6.2–7.9 with an optimum at pH 7.1. No growth occurred at pH lower than pH 6.0 or higher than pH 8.15. Strain Es71-Z0220T was halotolerant and grew at NaCl concentrations ranging from 0 to 18 g/L with an optimum at 6–8 g/L. No growth was evident at 20 g/L NaCl (Fig. S3). Growth occurred at sodium bicarbonate concentrations ranging from 0 to 20 g/L with an optimum at 7–8 g/L (Fig. S3). Minimal doubling time comprised 3 h under optimal growth conditions (47 °C, рН 7.1; 10 mM fumarate, acetate; 0.3 g/L Na2S·9H2O; 7 g/L NaH-CO3; 6.0 g/L NaCl).
Strain Es71-Z0220T oxidized acetate, lactate, pyruvate, succinate (Table 2), while molecular hydrogen, methanol, ethanol, n-propanol, butanol, formate, malate, citrate, N-acetyl-D-glucosamine, carboxymethyl- and microcrystalline cellulose were not oxidized and did not support the growth of the organism in the presence of external electron acceptors. Fermentative growth of strain Es71-Z0220T was not observed with peptone, yeast extract, beef extract, tryptone, glucose, sucrose, or cellobiose. The organism utilized SF, fumarate, nitrate, and elemental sulfur as electron acceptors with acetate being the electron donor. AQDS, sulfate, sulfite, thiosulfate, dimethylsulfoxide, selenite, crotonate, as well as soluble Fe(III) forms ferric citrate and Fe(III)-EDTA did not support respiratory growth of the organism. Strain Es71-Z0220T reduced SF to a black magnetic precipitate (Fig. 2a), producing 46 mM Fe(II) concomitantly with the oxidation of 15 mM acetate (Fig. 3). Growth on nitrate was accompanied by the formation of ca. 0.2–0.4 mM nitrite as an intermediate and finally, led to the production of 3.95 ± 0.25 mM ammonium by the stationary growth phase. Growth on elemental sulfur with acetate was sufficiently weaker than that on SF, fumarate, or nitrate (Table 2). However, the production of 2.76 ± 0.24 mM sulfide was detected by the stationary phase of growth on sul-fur.
A culture of strain Es71-Z0220T grown with synthetic ferrihydrite: (a) culture bottles, initial bottle with sterile medium and SF (left) vs. incubated bottle with SF transformed to black magnetic precipitate (right), note the clot of the precipitate at the bottle wall, outside which a neodymium hand magnet is attached; (b) fluorescence image of acridine orange-stained colony grown on a mineral particle of SF. Scale bar = 10 µm.
Strain Es71-Z0220T was resistant to the inhibitor of ribosomal protein biosynthesis kanamycin (100 mg/L) and sensitive to several other types of antibiotics. Its growth was ceased by the inhibitor of ribosomal protein biosynthesis neomycin (10 mg/L) and the inhibitors of cell wall biosynthesis (80 mg/L vancomycin or 100 mg/L ampicillin), as well as by the inhibitor of DNA-directed RNA polymerase rifampicin (0.1 mg/L).
Composition of cellular fatty acids, polar lipids, and quinones, redox spectrum of cytochromes. Prevailing fatty acids of the lipid membrane of strain Es71-Z0220T were the following saturated long- and branched-chain fatty acids: 9С-С14:0, C16:0, C18:0, iso-C14:0, 14С-15:0 (Tables 2 and S1). The main isoprenoid menaquinone of strain Es71-Z0220T was MK-8 detected previously in the representative of Deferribacterales Calditerrivibrio nitroreducens and Petrothermobacter organivorans (Iino et al., 2008; Tamazawa et al., 2017). Biomass of strain Es71-Z0220T during the growth on fumarate or nitrate, or being washed from large magnetite particles had pink to brownish color indicating active production of cytochromes. Suspension of cells, grown with fumarate, in a fresh anaerobic culture medium revealed a characteristic absorbance peak of oxidized c-type cytochromes at 405 nm (Fig. 4). Dithionite addition to the suspension returned peaks at 419, 522, and 552 nm in a redox difference absorption spectrum, which is characteristic of reduced c-type cytochromes. No reoxidation of the cytochromes was observed with further addition of soluble ferric(III) citrate and no peculiar absorbance peaks were detected in the fresh culture medium.
Spectrophotometric characterization of c-type cytochromes in the whole-cell suspension of D. essentukiensis Es71-Z0220T. Redox spectra of native (oxidized) c-type cytochromes, same cytochromes after their reduction with sodium dithionite (Red), and reduced versus oxidized difference spectrum (Red vs. Ox) are presented. Characteristic absorbance peaks of oxidized (at 405 nm) and reduced (at 419, 522, and 552 nm) c-type cytochromes are marked. The dashed line traces the background spectrum of uninoculated culture medium incubated for the same time as the grown culture. All the spectra are background corrected. Spectra of oxidized and reduced cytochromes and the background control are presented at the same scale, while the difference spectrum (green line) is given at a separate scale versus the supplementary Y-axis. AU – absorbance units.
Genome sequencing and phylogenetic analysis. The draft genome assembly of strain Es71-Z0220T resulted in 65 contigs with a total size of 2 362 387 bp and the genomic DNA G+C content of 34.04%. By using NCBI PGAP, the genome was predicted to contain 2 363 genes: 2 293 protein-coding sequences, 47 RNA genes including 3 rRNA and 40 tRNA, and 23 pseudogenes. We have analyzed the genomicdata concerning the phylogenetic position of strain Es71-Z0220T, its central carbon and energy metabolism, as well as its motility and putative chemotaxis.
Strain Es71-Z0220T has one rRNA operon. A comparison of 16S rRNA full length sequence with those available in GenBank (Benson et al., 1999) database showed that strain Es71-Z0220T is only distantly related to Deferribacteraceae family and has the highest pairwise identity to Petrothermobacter organivorans ANAT (94.05% identity) as well as to Calditerrivibrio nitroreducens DSM 19672T (89.64%) and Deferribacter thermophilus BMA1T (88.08%). The average amino acid identity (AAI) of D. essentukiensis and other sequenced representatives of Deferribacterales comprised from 48 to 59% (Fig. S4). To clarify the taxonomic rank of strain Es71-Z0220T, phylogenetic reconstruction was performed basing on concatenated partial amino acid sequences of 120 bacterial conservative proteins (Fig. 5). This analysis showed that strain Es71-Z0220T is clearly separated from other known genera and families of Deferribacteres class and forms a distinct family-level lineage within the Deferribacterales order. Considering these results and basing on GTDB (Parks et al., 2020), we propose that strain Es71-Z0220T represents a novel species of a new genus in a new family with the name Deferrivibrio essentukiensis gen. nov., sp. nov. Considering relatively high pairwise identity of 16S rRNA gene sequence of the strain Es71-Z0220T to Petrothermobacter organi-vorans ANAT (94.05% identity), we assume that these two microorganisms may belong to the same family-level lineage within the Deferribacterales order but unfortunately, the genome of the P. organivorans ANAT is not available that doesn’t allow us to test the proposal.
Placement of D. essentukiensis Es71-Z0220T within the Deferribacterales order based on phylogenetic analysis of concatenated partial amino acid sequences of 120 bacterial conservative proteins (Parks et al., 2020) by maximum likelihood inference. Bootstrap values above 90% are shown at the nodes. Bar, 0.10 changes per position. All phylogenetic designations are based on GTDB (Parks et al., 2021).
Genome Analysis
Central metabolism. The proton-translocating type I NADH-dehydrogenase (complex I of the respiratory electron transfer chain) is encoded in locus LF845_01775-01845 in the order nuoNMLKJIHGFEDCBA. The respiratory complex II (succinate dehydrogenase/fumarate reductase) is encoded in locus LF845_01775-01845. Oxidative phosphorylation in strain Es71-Z0220T is determined by a typical F0F1-ATP-synthase, which subunits are encoded by two remote gene clusters. The genes of cytoplasmic subunits ε, β, γ, α, δ, and B are located in cluster LF845_01490-01520, and the genes of the membrane stator subunit A and the building block of the membrane rotor subunit C are included in gene cluster LF845_08945-08950 together with a regulatory subunit AtpZ, proposed to inhibit the dephosphorylating activity of ATPase and favor the ATP synthesis (Mendoza-Hoffmann et al., 2018). Genome of strain Es71-Z0220T harbors key genes of acetate and pyruvate metabolism—acetyl-CoA synthetase (LF845_04900) and pyruvate carboxylase (LF845_11100). Succinate and lactate are likely to enter the metabolic pathways of D. essentukiensis via malate/L-lactate dehydrogenase (LF845_02525) and succinate dehydrogenase (LF845_02470-02490), involved in TCA cycle turnover. All the other key enzymes of this cycle are encoded adjacently to these dehydrogenases in the locus LF845_02495-02540, with the exception of the citrate synthase which is encoded by LF845_10495. Carbon substrates uptake in D. essentukiensis is determined by TRAP-family pyruvate and lactate transporters LF845_04590-04600 and LF845_03890-03910, as well as by putative succinate-acetate/proton symporters LF845_07175 and LF845_06765.
Fe(III) respiration. Screening of D. essentukiensis genome revealed 18 genes encoding various c-type multiheme cytochromes with 2−28 conserved heme-binding motifs per protein sequence. All of these multihemes are predicted to be secreted or membrane-anchored proteins facing the periplasm. Accordingly, any of the multihemes could be involved in extracellular electron transfer to insoluble acceptors, such as ferrihydrite and other Fe(III) minerals. Among these multihemes, ten proteins share considerable homology with previously characterized parts of EET chains in iron-reducing bacteria (Shi et al., 2016; Costa et al., 2019; Gavrilov et al., 2021) (Table S2). Cytochromes LF845_03595 and LF845_05585 are homologous to phylogenetically related putative terminal Fe(III) reductases OcwA of ‘T. potens’ and OmhA of Carboxydothermus ferrireducens (Costa et al., 2019; Gavrilov et al., 2021). Cytochromes LF845_01595 and LF845_09875 are homologous to SmhA octaheme of C. ferrireducens, and LF845_05580 is homologous to heptaheme cytochrome Ga0395992_02_30996_31874 of this same organism. Both homologs from C. ferrireducens are upregulated upon growth with insoluble electron acceptors (Gavrilov et al., 2021). Putative membrane-anchored cytochromes LF845_05495 and LF845_05525 of D. essentukiensis share equally low homology with OmcE outer cell surface cytochrome of G. sulfurreducens (Shi et al., 2016). Cytochrome LF845_05545 is homologous to DmsE periplasmic electron shuttle of S. oneidensis, while the largest, 15‑ and 28-heme, cytochromes of D. essentukiensis LF845_05555 and LF845_05560 share homology with MtrA periplasmic decaheme of EET-driving porin-cytochrome complex of S. oneidensis (Schickberger et al., 2013). Eight of the mentioned EET-related multihemes of D. essentukiensis are encoded by colocalized genes in the locus LF845_05495-05585 together with two more cytochromes. Five of the cytochromes encoded in the locus LF845_05495-05585 contain putative haematite-binding motifs TPT, SPT, and TVTPS (Lower et al., 2008). In LF845_05585 and LF845_05545 protein sequences, such motifs are located between two predicted heme-binding sites. Detailed characteristics of the identified multiheme cytochromes are given in Table S2. The “cytochrome locus” of D. essentukiensis also encodes putative menaquinol-oxidizing cytochrome b/b6 domain-containing protein LF845_05575 and an NHL repeat-containing protein LF845_05565. Blast analysis of the identified EET-related genes of D. essentukiensis versus previously described ones from the Fe(III)-reducing Deferribacter autotrophicus and non-iron-reducing D. desulfuricans (Slobodkin et al., 2019) revealed considerable homology of the majority of multiheme cytochromes in all the three microorganisms of the order Deferribacterales. In addition, similar arrangement of homologous genes encoding 9 multihemes, the cytochrome b/b6 domain-containing protein and the NHL repeat-containing protein was observed in the “cytochrome locus” and clusters of cytochrome-encoding genes from Deferribacter autotrophicus and D. desulfuricans (Slobodkin et al., 2019). Interestingly, only the Fe(III)-reducing microorganisms Deferrivibrio essentukiensis and Deferribacter autotrophicus possess homologous SmhA-related multihemes (LF845_01595 and LF845_09875 in D. essentukiensis). We did not identify any considerable homologs of these proteins, or SmhA multiheme from C. ferrireducens, in the non-iron-reducing Deferri-bacter desulfuricans.
Nitrate and sulfur respiration. D. essentukiensis possesses a gene cluster encoding nitrate-reducing Nap-system proteins NapHGDA in this particular sequence (LF845_02950-02965). Putative redox partner of the periplasmic molybdopterin nitrate reductase NapA, the tetraheme cytochrome NapM, is encoded adjacently by LF845_02970, while a homolog of menaquinone-oxidizing membrane-bound cytochrome of NapC/NrfH family is encoded remotely by LF845_08935. Genome of D. essentukiensis lacks the genes of NrfAH ammonium-forming nitrite-reducing complex but encodes a homolog of an octaheme c hydroxylamine oxidoreductase of εHao group (Simon and Klotz, 2013) adjacently to the “Nap locus” (LF845_02975). Strain Es71-Z0220T possesses the genes of several catalytic subunits of molybdopterin oxidoreductase complexes, one of which share the closest homology with polysulfide/thiosulfate reductase subfamily proteins and is encoded adjacently to typical Fe-S and membrane-bound subunits of CISM family oxidoreductases (Rothery et al., 2008) in the locus LF845_11445-11455.
Genes related to oxygen respiration or tolerance. D. essentukiensis possesses an operon encoding a cbb3-type heme-copper cytochrome c oxidase (LF845_03180-03210). However, any genes of redox partners of terminal cytochrome c oxidases, such as canonical or alternative respiratory complexes III, are absent from D. essentukiensis genome. Also, catalase genes were not detected in the genome.
Motility and chemotaxis. D. essentukiensis possesses complete gene sets determining flagellum assembly and chemotaxis. The genes of flagellum assembly are localized in several genomic regions: locus LF845_00175-00225 encodes major proteins of the basal body, flagellar rod, and hook, locus LF845_02070-02105 encodes type III secretion system proteins and other factors of flagellar biosynthesis, locus LF845_07640-07685 encodes flagellar biosynthesis proteins including H+-dependent stator MotAB and the motor switch protein FliM. Interestingly, genes of the flagellar filament fliDST are remote from the mentioned loci, the major flagellin FliC is also encoded remotely by LF845_07440. Taxis genes, homologous to previously proposed determinants of redox-mediated chemotaxis in Fe(III)-reducing Shewanella species (Starwalt-Lee et al., 2021), are grouped in the locus LF845_06375-06460 of D. essentukiensis genome. This gene set encodes CheA histidine kinase (LF845_06410) and CheBRYZ regulatory proteins which altogether are proposed to transduce the signal from activated methyl-accepting chemotaxis proteins (MCP receptors) to the motor switch protein FliM (LF845_06430), regulating the direction of flagellar rotation. Additionally, this locus encodes the sigma factor FliA which controls the expression of flagella-related genes. Several MCP receptor proteins and the CheW protein (LF845_04070), providing structural support for MCP arrays, are encoded remotely from the “chemotaxis locus”.
DISCUSSION
The order Deferribacterales (Huber et al., 2001) of Deferribacterota phylum (Whitman et al., 2018) currently includes one validly described family Deferri-bacteraceae which consists of eight genera: Calditerrivibrio (Iino et al., 2008), Flexistipes (Fiala et al., 1990), Deferribacter (Greene et al., 1997), Denitrovibrio (Myhr and Torsvik, 2000), Petrothermobacter (Tamazawa et al., 2017), Geovibrio (Caccavo et al., 1996), Seleniivibrio (Rauschenbach et al., 2013), and Mucispirillum (Robertson et al., 2005). However, according to genome-based GTDB phylogeny, the order includes 4 families and does not harbor the genus Mucispirillum, which branches off the other known genera of this order (Fig. 5). With the exception of M. schaedleri, which was isolated from gastrointestinal mucus of mice, all the members of this order are free-living bacteria isolated from natural thermal waters, petroleum reservoirs, deep oceanic sediments. Most of these bacteria are strictly anaerobic, halotolerant, moderately thermophilic, and neutrophilic Gram-negative vibrios with a respiratory type of metabolism. All members of the order Deferribacterales, except for fermentative bacterium F. sinusarabici, can utilize acetate and a wide spectrum of other organic acids, as well as peptides, amino acids, and alcohols as electron donors. Typical electron acceptors for these bacteria are Fe(III) in soluble or insoluble forms, Mn(IV), elemental sulfur, or nitrate. Among the members of the order, only P. organivorans is able to reduce sulfate (Tamazawa et al., 2017). Representatives of Deferribacter and Geovibrio genera can oxidize molecular hydrogen upon respiratory growth in the absence of organic carbon, thus being chemolithoautotrophs. Phenotypic characteristics of strain Es71-Z0220T generally correspond to those described for the majority of cultured Deferribacterales (Table 2). The novel isolate Es71-Z0220T is strictly anaerobic, moderately thermophilic, neutrophilic halotolerant gram-negative vibrio, capable of dissimilatory reduction of ferrihydrite, nitrate, fumarate, and elemental sulfur with acetate or other organic acids as electron donors. Its optimal physico-chemical growth conditions perfectly correlate with those, characteristic for Yessentuki no. 4 type mineral water. The peculiar feature of the isolate Es71-Z0220T is its inability to reduce soluble Fe(III) compounds which correlates with the absence of redox interaction between its c-type cytochromes and Fe(III) citrate complex (Fig. 4). Such physiological feature might reflect adaptation of this Fe(III)-reducing organism to the life in pores and fractures of water-bearing sedimentary rocks of the slightly alkaline aquifer in which iron solubility is extremely low and insoluble iron minerals represent the most widespread form of Fe(III) available for anaerobic respiration. Multiheme c-type cytochromes are the most likely determinants of Fe(III)-reducing activity in strain Es71-Z0220T, analogously to the majority of currently described iron reducing prokaryotes (Faustino et al., 2021; Gavrilov et al., 2021). The organism possesses a set of 18 c-type multiheme genes, 11 of which are co-localized in the genome. Seven proteins encoded in the “cytochrome locus’” are predicted to be secreted or membrane-bound periplasm-facing cytochromes, sharing considerable homology with previously described determinants of EET pathways. Amino acid sequences of five of these cytochromes contain putative haematite-binding motifs, several of which are flanked with heme-binding sites, similarly to MtrC outer membrane cytochrome driving Fe(III) reduction in S. oneidensis (Lower et al., 2008). The multihemes with these motifs are the most probable terminal Fe(III) reductases. The “cytochrome locus” of D. essentukiensis also encodes putative menaquinol-oxidizing cytochrome b/b6 and an NHL repeat-containing protein, which might bind mineral nanocrystals of transition metal compounds (Voet et al., 2015). Accordingly, the locus LF845_05495-05585 is likely to be the major determinant of the electron transfer pathway from the quinone pool to extracellular electron acceptors in D. essentukiensis. Putative terminal reductases of this pathway are likely to have high affinity to insoluble Fe(III) forms, analogously to previously described cytochromes of an obligate Fe(III) reducer Geoglobus acetivorans (Mardanov et al., 2015). However, we should clarify that the “cytochrome locus” per se does not determine the D. essentukiensis’s capability of EET. Gene clusters, encoding the homologs of all the above mentioned proteins of this locus, with similar gene arrangement were previously identified in two physiologically different representatives of Deferribacterales order, i.e., in the iron reducer D. autotrophicus, and in D. desulfuricans, which is incapable of Fe(III) reduction (Slobodkin et al., 2019). Our comparative analysis of multiheme cytochromes repertoire of these two organisms and Deferrivibrio essentukiensis revealed that only iron reducing D. autotrophicus and Deferri-vibrio essentukiensis possess the homologs of an octaheme cytochrome SmhA of C. ferrireducens. We have previously demonstrated sufficient upregulation of this cytochrome expression in the presence of insoluble electron acceptors, as well as its phylogenetic relation with the octaheme cytochrome FHQ18_RS08740 (WP_149266789.1) of Deferribacter autotrophicus (Gavrilov et al., 2021). So, the homologs of SmhA are highly likely to be the key determinants of iron reduction in different representatives of Deferribacterales.
The pathway for nitrate reduction in Deferrivibrio essentukiensis is similar to those previously identified in several ammonifying bacteria of Deferribacteres phylum (Slobodkin et al., 2019). Genome of D. essentukiensis contains the genes of nitrate reducing Nap-complex. However, instead of the archetypal complex of the ammonifying nitrite reductase NrfAH, D. essentukiensis possesses an octaheme hydroxylamine oxidoreductase from εHao phylogenetic clade. This group of cytochromes has been previously proposed to play the key role in nitrite to ammonium reduction in the absence of NrfAH in Epsilonproteobacteria (Simon and Klotz, 2013), bacteria of Calditrichaeota phylum (Kublanov et al., 2017) and some others.
Genomic determinants of central carbon and energy metabolism, as well as the pathways for organic substrates utilization in D. essentukiensis are rather typical for strictly anaerobic chemoorganoheterotrophs. The organism possesses proton-translocating complex I and complex II fumarate reductase of the respiratory electron transfer chain, an archetypal F0F1-type ATP-synthetase, several transmembrane quinol-oxidizing Fe-S- and heme-containing proteins, such as the cytochrome b/b6, PsrC of putative polysulfide reductase complex, NapC and NapH proteins, which couple the generation of proton motive force with Fe(III), sulfur and nitrate respiration, respectively. All the necessary transporters of key carbon substrates and electron donors of D. essentukiensis (acetate, pyruvate, succinate, and lactate) are encoded in its genome as well as the enzymes necessary for the oxidation of these substrates and their utilization as the energy sources via the TCA cycle.
Interestingly, the genome of this organism encodes a cbb3-type heme-copper cytochrome oxidase, which is proposed to drive oxygen respiration under microaerobic growth conditions (Pitcher et al., 2002). At this, the inability of the organism for microaerobic or aerobic growth could be explained by the lack of respiratory complex III genes which are indispensable for oxygen respiration.
Taken together, the metabolic features of D. essentukiensis could define its important ecological role in the subsurface ecosystem as the microorganism coupling the oxidation of organic matter with redox transformation of Fe(III) minerals in water-bearing sediments of the aquifer. Environmental significance of D. essentukiensis seems doubtful if one considers the extremely low representation of Deferribacterales-related phylotypes in fresh mineral water samples. In this view, the novel species could rather belong to the “rare biosphere” of the ecosystem. The phenomenon of “rare biosphere” combines small subpopulations of prokaryotes, each comprising less than 1% of 16S rRNA gene library of a microbial community, into a single ecologically relevant unit (Skopina et al., 2016; Jousset et al., 2017). To date, minor groups of microorganisms are regarded to serve as a source of crucial genetic material for a microbial community, activated under sharp changes of environmental conditions (Jousset et al., 2017), to be the first inhabitants of newly appeared or completely modified ecological niches (Skopina et al., 2016), and to support the content of important growth factors, such as vitamins or organic nitrogen, at low but critically needed level (Sohm et al., 2011; Zhang et al., 2019).
An alternative explanation of D. essentukiensis’ low representation in the microbial community lies in possibly uneven outwash of microorganisms from the pores of water-bearing rocks during drilling and extraction of mineral water. That is especially important for the organisms which depend on insoluble substrates, such as Fe(III) minerals, and are likely to form biofilms on the mineral surfaces. We did observe dense colonies of D. ferrireducens cells grown with SF (Fig. 2b). In this view, flagellum-driven motility of the organism could reflect its ability for redox-sensitive chemotaxis, including possible tactic migration to insoluble electron acceptors which is extensively studied within the past decade in dissimilatory metal reducing and electrogenic bacteria (Starwalt-Lee et al., 2021). The ability of D. ferrireducens to sense and locate insoluble electron acceptors correlates with the fact, that its chemotaxis-related genes, clustered together in the locus LF845_06375-06460, share homology with those recently identified in Fe(III)-reducing and electrogenic bacteria of Shewanella genus (Starwalt-Lee et al., 2021). Isolation of the second Fe(III)-reducing strain Es71N of D. ferrireducens from water of the same aquifer, sampled via the backup well 71N with a nine-month delay, indicates the persistence of the novel species in the studied environment. This fact makes equiprobable both of the mentioned assumptions: (1) the hypothesis on the abundance of D. ferrireducens in the sampled Cretaceous aquifer and its uneven outwash from biofilms covering the water-bearing rocks, and (2) the assumption on D. essentukiensis belonging to the “rare biosphere” of the environment, which representatives can rapidly outnumber other members of the microbial community upon favorable changes of physico-chemical conditions. Clearly, further studies are needed to clarify the ecological role of the novel isolate in its natural environment and determine the exact impact of its metabolic activity on the formation of the composition of YMWD mineral waters.
On the basis of physiological properties and phylogenetic analysis, we propose strain Es71N-Z0220T as the type strain of a new species and new genus under the name Deferrivibrio essentukiensis gen. nov., sp. nov., being the first representative of the new family Deferrivibrionaceae fam. nov.
Description of Deferrivibrionaceae fam. nov.
Deferrivibrionaceae (De.fer.ri.vi.bri.o.na.ce’ae. N.L. masc. n. Deferrivibrio), type genus of the family; L. fem. pl. suff. -aceae, ending to denote a family; N.L. fem. pl. n. Deferrivibrionaceae, the Deferrivibrio family.
The family is established basing on phylogenetic analysis of 120 bacterial conservative marker genes, as well as on morpho-physiological characteristics. Gram-negative cells appear as vibrio-shaped rods and do not form spores. The family includes anaerobic chemoorganotrophic species. The type genus is Deferrivibrio.
Description of Deferrivibrio gen. nov.
Deferrivibrio (De.fer.ri.vi’bri.o. L. pref. de, from; L. neut. n. ferrum, iron; L. v. vibrare vibrate; N.L. masc. n. vibrio), name of bacteria possessing a curved rod shape, that vibrates; N.L. masc. n. Deferrivibrio, a vibrio that reduces iron.
Cells are motile non-sporeforming gram-negative vibrio-shaped rods. Anaerobe. Positive for catalase and oxidase. Neutrophile and thermophile. Chemoorganotrophic with the primarily organic acid oxidation metabolism. Capable of iron-, nitrate- and sulfur reduction. Cellular fatty acids are composed mainly of 14−18-carbon-containing saturated long- and branched-chain fatty acids. The main isoprenoid menaquinone is MK-8.
The type species is Deferrivibrio essentukiensis sp. nov.
Description of Deferrivibrio essentukiensis sp. nov.
Essentukiensis (es.sen.tu.ki.en’sis. N.L. masc. adj. essentukiensis, from New Latin transliteration of Yessentuki), named after the place of origin, Yessentukskoye mineral water deposit, Russia.
Cells are non-sporeforming, motile by means of one polar flagellum, vibrio-shaped, 0.4–0.5 μm in width and 1–3 μm in length. The cell wall of gram-negative structure. Moderate thermophile, growth temperature ranges from 30 to 54°C, with an optimum at 47°C. Neutrophile with growth pH ranging from 6.2 to 7.9, with an optimum at pH 7.1. Halotolerant, growing at NaCl concentrations ranging from 0 to 18 g/L, with an optimum at 7 g/L. Anaerobic, but catalase and oxidase positive. Chemoorganotroph, capable of growth using acetate, lactate, pyruvate, or succinate as electron donors with ferrihydrite as the electron acceptor. Unable to oxidize molecular hydrogen, methanol, ethanol, n-propanol, butanol, formate, malate, citrate, microcrystalline- and carboxymethylcellulose, starch, pectin, yeast extract, beef extract, tryptone, casamino acids with ferrihydrite as the electron acceptor. Cannot ferment peptone, yeast extract, beef extract, tryptone, cellobiose, glucose, or sucrose. With acetate as the electron donor can reduce fumarate, nitrate, and elemental sulfur, but not AQDS, Fe(III)-citrate, Fe(III)-EDTA, thiosulfate, sulfate, sulfite, dimethylsulfoxide, nitrite, selenate, or crotonate. The main fatty acids are 9С-С14:0, C16:0, C18:0, iso-C14:0, 14С-15:0. The main isoprenoid menaquinone is MK-8. Highly sensitive to rifampicin antibiotic, inhibiting DNA-directed RNA polymerase, less sensitive to the inhibitors of ribosomal protein biosynthesis and cell wall biosynthesis. The type strain is Es71-Z0220T (=VKM B-3557 = JCM 39245), which was isolated from subsurface mineral water of Yessentukskoye mineral water deposit, well 71.
All the sequencing data are deposited in NCBI BioProject PRJNA753547.
The paper contains Supplementary materials: Tables S1 and S2, Figs. S1, S2, S3, and S4.
Change history
23 September 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S0026261722700011
03 June 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S002626172213001X
REFERENCES
Abramov, V.Yu. and Vavichkin, A.Yu., Characteristics of formation of the thermogasochemical composition of the Yessentuki deposit mineral waters, Razvedka i Okhrana N-edr, 2010, no. 10, pp. 27‒32.
Aklujkar, M., Coppi, M.V., Leang, C., Kim, B.C., Chavan, M.A., Perpetua, L., Giloteaux, L., Liu, A., and Holmes, D.E., Proteins involved in electron transfer to Fe(III) and Mn(IV) oxides by Geobacter sulfurreducens and Geobacter uraniireducens, Microbiology (SGM), 2013, vol. 159, pp. 515–535.
Anisimova, M. and Gascuel, O., Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative, Syst. Biol., 2006, vol. 55, pp. 539–552.
Benson, D., Boguski, M., Lipman, D., Ostell, J., Ouellette, B., Rapp, B.A., and Wheeler, D.L., GenBank, Nucl. Acids Res., 1999, vol. 27, pp. 38–43.
Butler, J.E., Young, N.D., and Lovley, D.R., Evolution of electron transfer out of the cell: comparative genomics of six Geobacter genomes, BMC Genomics, 2010, vol. 11, р. 40.
Caccavo, F.J., Coates, J.D., Rossello-Mora, R.A., Ludwig, W., Schleifer, K.H., Lovley, D.R., and McInerney, M.J., Geovibrio ferrireducens, a phylogenetically distinct dissimilatory Fe(III)-reducing bacterium, Arch. Microbiol., 1996, vol. 165, pp. 370–376.
Cappuccino, J.G. and Sherman, N., Microbiology: A Laboratory Manual, San Francisco: Benjamin 302 Cummings Pearson Education, 2002, 6th ed.
Carlson, H.K., Iavarone, A.T., Gorur, A., Yeo, B.S., Tran, R., Melnyk, R.A., Mathies, R.A., Auer, M., and Coates, J.D., Surface multiheme c-type cytochromes from Thermincola potens and implications for respiratory metal reduction by Gram-positive bacteria, Proc. Natl. Acad. Sci. U. S. A., 2012, vol. 109, pp. 1702–1707.
Collins, M.D., Analysis of isoprenoid quinones, Methods Microbiol., 1985, vol. 18, pp. 329–363.
Collins, M.D. and Jones, D., Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications, Microbiol. Rev., 1981, vol. 15, pp. 316–354.
Costa, N.L., Hermann, B., Fourmond, V., Fausti-no, M.M., Teixeira, M., Einsle, O., Paquete, C.M., and Louro, R.O., How thermophilic Gram-positive organisms perform extracellular electron transfer: characterization of the cell surface terminal reductase OcwA, mBio, 2019, vol. 10, e01210-19.
Coursolle, D. and Gralnick, J.A., Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1, Mol. Microbiol., 2010, vol. 77, pp. 995–1008.
Faustino, M.M., Fonseca, B.M., Costa, N.L., Lousa, D., Louro, R.O., and Paquete, C.M., Crossing the wall: characterization of the multiheme cytochromes involved in the extracellular electron transfer pathway of Thermincola ferriacetica, Microorganisms, 2021, vol. 9, art. 293.
Fiala, G., Woese, C.R., Langworthy, T.A., and Stetter, K.O., Flexistipes sinusarabici, a novel genus and species of eubacteria occurring in the Atlantis II Deep brines of the Red Sea, Arch. Microbiol., 1990, vol. 154, pp. 120–126.
Filimonova, E., Kharitonova, N., Sartykov, A., Maximova, E., Baranovskaya, E., Korzun, A., Maslov, A., Baidariko, E., and Lavrushin, V., Hydrogeology and hydrogeochemistry of mineral sparkling groundwater within Yessentuki area (Caucasian Mineral Water region), Environ. Earth Sci., 2020, vol. 79, p. 15.
Gavrilov, S.N., Zavarzina, D.G., Elizarov, I.M., Tikhonova, T.V., Dergousova, N.I., Popov, O., Lloyd, J.R., Knight, D., El-Naggar, M.Y., Pirbadian, S., Leung, K.M., Robb, F.T., Zakhartsev, M.V., Bretschger, O., and Bonch-Osmolovskaya, E.A., Novel extracellular electron transfer channels in a Gram-positive thermophilic bacterium, Front. Microbiol., 2021, vol. 11, art. 3349.
Gohl, D.M., MacLean, A., Hauge, A., Becker, A., Walek, D., and Beckman, K.B., An optimized protocol for high-throughput amplicon-based microbiome profiling, Protoc. Exch., 2016. https://doi.org/10.1038/protex.2016.030
Greene, A.C., Patel, B.K., and Sheehy, A.J., Deferribacter thermophiles gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir, Int. J. Syst. Bacteriol., 1997, vol. 47, pp. 505–509.
Härtig, C., Rapid identification of fatty acid methyl esters using a multidimensional gas chromatography-mass spectrometry database, J. Chromatogr., 2008, vol. 1177, pp. 159–169.
Hoang, D.T., Chernomor, O., von Haeseler, A., Minh, B.Q., and Vinh, L.S., UFBoot2: improving the ultrafast bootstrap approximation, Mol. Biol. Evol., 2018, vol. 35, pp. 518–522.
Huber, H. and Stetter, K.O., Order, I. Deferribacterales ord. nov., in Bergey’s Manual of Systematic Bacteriology, The archaea and the deeply branching and phototrophic bacteria, Boone, D.R., Castenholz, R.W., and Garrity, G.M., Eds., New York: Springer, 2001, vol. 1, 2nd ed., pp. 465–471.
Hugerth, L.W., Wefer, H.A., Lundin, S., Jakobsson, H.E., Lindberg, M., Rodin, S., Engstrand, L., and Anders-son, A.F., DegePrime, a program for degenerate primer design for broad-taxonomic-range pcr in microbial ecology studies, Appl. Environ. Microbiol., 2014, vol. 80, pp. 5116–5123.
Iino T, Nakagawa T, Mori K, Harayama S, Suzuki K. Calditerrivibrio nitroreducens gen. nov., sp. nov., a thermophilic, nitrate-reducing bacterium isolated from a terrestrial hot spring in Japan // Int. J. Syst. Evol. Microbiol. 2008. vol. 58. pp. 1675-1679.
Jousset, A., Bienhold, C., Chatzinotas, A., Gallien, L., Gobet, A., Kurm, V., Küsel, K., Rillig, M.C., and Ri-vett, D.W., Where less may be more: how the rare biosphere pulls ecosystems strings, ISME J., 2017, vol. 11, pp. 853–862.
Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., von Haeseler, A., and Jermiin, L.S., ModelFinder: fast model selection for accurate phylogenetic estimates, Nat. Methods, 2017, vol. 14, pp. 587–589.
Kevbrin, V.V. and Zavarzin, G.A., The influence of sulfur compounds on the growth of halophilic homoacetic bacterium Acetohalobium arabaticum, Microbiology (Moscow), 1992, vol. 61, pp. 563–571.
Krauze, P., Kämpf, H., Horn, F., Liu, Q., Voropaev, A., Wagner, D., and Alawi, M., Microbiological and geochemical survey of CO2-dominated mofette and mineral waters of the Cheb Basin, Czech Republic, Front. Microbiol., 2017, vol. 8, art. 2446.
Kublanov, I.V., Sigalova, O.M., Gavrilov, S.N., Lebedinsky, A.V., Rinke, C., Kovaleva, O., Chernyh, N.A., Ivanova, N., Daum, C., Reddy, T.B.K., Klenk, H.-P., Spring, S., Göker, M., Reva, O.N., Miroshnichenko, M.L., et al., Genomic analysis of Caldithrix abyssi, the thermophilic anaerobic bacterium of the novel bacterial phylum Calditrichaeota, Front. Microbiol., 2017, vol. 8, art. 195.
Lavrushin, V.Y., Lisenkov, A.B., and Aidarkozhina, A.S., Genesis of the Yessentuki deposit of carbonated waters, North Caucasus, Geochem. Int., 2020, vol. 58, pp. 77–90.
Leclerc, H. and da Costa, M.S., Microbiology of natural mineral waters, in Technology of Bottled Water, Senior, D. and Dege, N., Eds., Blackwell, 2005, 2nd edn., pp. 325–387.
Lesaulnier, C.C., Herbold, C.W., Pelikan, C., Berry, D., Gérard, C., Le Coz, X., Gagnot, S., Niggemann, J., Dittmar, T., Singer, G.A., and Loy, A., Bottled aqua incognita: microbiota assembly and dissolved organic matter diversity in natural mineral waters, Microbiome, 2017, vol. 5, art. 126.
Lower, B.H., Lins, R.D., Oestreicher, Z., Straatsma, T.P., Hochella, M.F., Shi, L., and Lower, S.K., In vitro evolution of a peptide with a hematite binding motif that may constitute a natural metal-oxide binding archetype, Environ. Sci. Technol., 2008, vol. 42, pp. 3821–3827.
Loy, A., Beisker, W., and Meier, H., Diversity of bacteria growing in natural mineral water after bottling, Appl. Environ. Microbiol., 2005, vol. 71, pp. 3624–3632.
Mardanov, A.V., Slododkina, G.B., Slobodkin, A.I., Beletsky, A.V., Gavrilov, S.N., Kublanov, I.V., Bonch-Osmolovskaya, E.A., Skryabin, K.G., and Ravin, N., The Geoglobus acetivorans genome: Fe(III) reduction, acetate utilization, autotrophic growth, and degradation of aromatic compounds in a hyperthermophilic archaeon, Appl. Environ. Microbiol., 2015, vol. 81, pp. 1003–1012.
Mendoza-Hoffmann, F., Pérez-Oseguera, A., Ceval-los, M.A., Zarco-Zavala, M., Ortega, R., Peña-Segura, C., Espinoza-Simón, E., Uribe-Carvajal, S. and García-Trejo, J.J., The biological role of the ζ subunit as unidirectional inhibitor of the F1F0-ATPase of Paracoccus denitrificans, Cell Reports, 2018, vol. 22, pp. 1067–1078.
Merkel, A.Y., Chernyh, N.A., Pimenov, N.V., Bonch-Osmolovskaya, E.A., and Slobodkin, A.I., Diversity and metabolic potential of the terrestrial mud volcano microbial community with a high abundance of archaea mediating the anaerobic oxidation of methane, Life, 2021, vol. 11, art. 953.
Merkel, A.Y., Tarnovetskii, I.Y., Podosokorskaya, O.A., and Toshchakov, S.V., Analysis of 16S rRNA primer systems for profiling of thermophilic microbial communities, Microbiology (Moscow), 2019, vol. 88, pp. 671–680.
Myhr, S. and Torsvik, T., Denitrovibrio acetiphilus, a novel genus and species of dissimilatory nitrate-reducing bacterium isolated from an oil reservoir model column, Int. J. Syst. Evol. Microbiol., 2000, vol. 50, pp. 1611–1619.
Nguyen, L.T., Schmidt, H.A., von Haeseler, A., and Minh, B.Q., IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies, Mol. Biol. Evol., 2015, vol. 32, pp. 268–274.
Parks, D.H., Chuvochina, M., Chaumeil, P.A., Rinke, C., Mussig, A.J., and Hugenholtz, P.A., Complete domain-to-species taxonomy for Bacteria and Archaea, Nat. Biotechnol., 2020, vol. 38, pp. 1079–1086.
Pitcher, R.S., Brittain, T., and Watmough, N.J., Cytochrome cbb 3 oxidase and bacterial microaerobic metabolism, Biochem. Soc. Trans., 2002, vol. 30, pp. 653–658.
Potapov, E.G., Danilov, S.R., and Gadzhikhanova, S.U., Genesis of hydrocarbonate-sulfide mineral waters of the Yessentuki deposit according to the data of hydrochemical, microbiological, and isotopic studies, Kurort. Med., 2017, no. 1, pp. 11–16.
Potapov, E.G., Dubinina, G.A., Danilov, S.R., Gadzhikhanova, S.U., Shchelkunov, A.V., and Grabovicj, M.Yu., Physicochemical and microbiological investigation of deep mineral waters of the CMW area, Kurort. Med., 2014, no. 4, pp. 14–20.
Rauschenbach, I., Posternak, V., Cantarella, P., McConnell, J., Starovoytov, V., and Häggblom, M.M., Seleniivibrio woodruffii gen. nov., sp. nov., a selenate- and arsenate-respiring bacterium in the Deferribacteraceae, Int. J. Syst. Evol. Microbiol., 2013, vol. 63, pp. 3659–3665.
Robertson, B.R., O’Rourke, J.L., Neilan, B.A., Vandamme, P., On, S.L.W., Fox, J.G., and Lee, A., Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents, Int. J. Syst. Evol. Microbiol., 2005, vol. 55, pp. 1199–1204.
Rothery, R.A., Gregory, J., Workun, G.J., and Weiner, J.H., The prokaryotic complex iron–sulfur molybdoenzyme family, Biochim. Biophys. Acta, 2008, vol. 1778, pp. 1897–1929.
Sala-Comorera, L., Caudet-Segarra, L., Galofréc, B., Lucena, F., Blanch, A.R., and García-Aljaro, C., Unravelling the composition of tap and mineral water microbiota: divergences between next-generation sequencing techniques and culture based methods, Int. J. Food Microbiol., 2020, vol. 334, art. 108850.
Scheiner, D., Determination of ammonia and Kjeldahl nitrogen by indophenol method, Water Res., 1976, vol. 10, pp. 31‒36.
Schicklberger, M., Sturm, G., and Gescher, J., Genomic plasticity enables a secondary electron transport pathway in Shewanella oneidensis, Appl. Environ. Microbiol., 2013, vol. 79, pp. 1150–1159.
Shi, L., Dong, H., Reguera, G., Beyenal, H., Lu, A., Liu, J., Yu, H-Q., and Fredrickson, J.K., Extracellular electron transfer mechanisms between microorganisms and minerals, Nat. Rev. Microbiol., 2016, vol. 14, pp. 651–652.
Simon, J. and Klotz, M.G., Diversity and evolution of bioenergetic systems involved in microbial nitrogen compound transformations, Biochim. Biophys. Acta, 2013, vol. 1827, pp. 114–135.
Skopina, M.Yu., Vasileva, A.A., Pershina, E.V., and Pinevich, A.V., Diversity at low abundance: the phenomenon of the Rare Bacterial Biosphere, Microbiology (Moscow), 2016, vol. 85, pp. 272–282.
Slobodkin, A., Slobodkina, G., Allioux, M., Alain, K., Jebbar, M., Shadrin, V., Kublanov, I., Toshchakov, S., and Bonch-Osmolovskaya, E., Genomic insights into the carbon and energy metabolism of a thermophilic deep-sea bacterium Deferribacter autotrophicus revealed new metabolic traits in the phylum Deferribacteres, Genes, 2019, vol. 10, art. 849.
Sohm, J.A., Webb, E.A., and Capone, D.G., Emerging patterns of marine nitrogen fixation, Nat. Rev. Microbiol., 2011, vol. 9, pp. 499–508.
Starwalt-Lee, R., El-Naggar, M.Y., Bond, D.R., and Gralnick, J.A., Electrolocation? The evidence for redox-mediated taxis in Shewanella oneidensis, Mol. Microbiol., 2021, vol. 115, pp. 1069–1079.
Stookey, L.L., Ferrozine—a new spectrophotometric reagent for iron, Anal. Chem., 1970, vol. 42, pp. 779–781.
Tamazawa, S., Mayumi, D., Mochimaru, H., Sakata, S., Maeda, H., Wakayama, T., Ikarashi, M., Kamagata, Y., and Tamaki, H., Petrothermobacter organivorans gen. nov., sp. nov., a thermophilic, strictly anaerobic bacterium of the phylum Deferribacteres isolated from a deep subsurface oil reservoir, Int. J. Syst. Evol. Microbiol., 2017, vol. 67, pp. 3982–3986.
Tatusova, T., DiCuccio, M., Badretdin, A., Chetvernin, V., Nawrocki, E.P., Zaslavsky, L., Lomsadze, A., Pruitt, K.D., Borodovsky, M., and Ostell, J., NCBI prokaryotic genome annotation pipeline, Nucl. Acids Res., 2016, vol. 44, pp. 6614–6624.
Toshchakov, S.V., Lebedinsky, A.V., Sokolova, T.G., Zavarzina, D.G., Korzhenkov, A.A., Teplyuk, A.V., Chistyakova, N.I., Rusakov, V.S., Bonch-Osmolovskaya, E.A., Kublanov, I.V., and Gavrilov, S.N., Genomic insights into energy metabolism of Carboxydocella thermautotrophica coupling hydrogenogenic CO oxidation with the reduction of Fe(III) minerals, Front. Microbiol., 2018, vol. 9, art. 1759.
Trüper, H.G. and Schlegel, H.G., Sulphur metabolism in Thiorhodaceae I. Quantitative measurements on growing cells of Chromatium okenii, A. van Leeuwenhoek, 1964, vol. 30, pp. 225–238.
Voet, A.R., Noguchi, H., Addy, C., Zhang, K.Y., and Tame, J.R., Biomineralization of a cadmium chloride nanocrystal by a designed symmetrical protein, Angew. Chem. Int. Ed., 2015, vol. 54, pp. 9857–9860.
Whitman, W.B., Oren, A., Chuvochina, M., da Cos-ta, M.S., Garrity, G.M., Rainey, F.A., Rossello-Mora, R., Schink, B., Sutcliffe, I., Trujillo, M.E., and Ventura, S., Proposal of the suffix -ota to denote phyla. Addendum to “Proposal to include the rank of phylum in the International Code of Nomenclature of Prokaryotes”, Int. J. Syst. Evol. Microbiol., 2018, vol. 68, pp. 967–969.
Wick, R.R., Judd, L.M., Gorrie, C.L., and Holt, K.E., Unicycler: resolving bacterial genome assemblies from short and long sequencing reads, PLoS Comput. Biol., 2017, vol. 13. e1005595.
Wolin, E.A., Wolin, M.J., and Wolfe, R.S., Formation of methane by bacterial extracts, J. Biol. Chem., 1963, vol. 238, pp. 2882–2888.
Zavarzina, D.G., Kolganova, T.V., Boulygina, E.S., Kostrikina, N.A., Tourova, T.P., and Zavarzin, G.A., Geoalkalibacter ferrihydriticus gen. nov. sp. nov., the first alkaliphilic representative of the family Geobacteraceae, isolated from a soda lake, Microbiology (Moscow), 2006, vol. 75, pp. 673–682.
Zhang, Y., Shuikui, D., Qingzhu, G., Ganjurjav, H., Xu-exia, W., and Wei, G. “Rare biosphere” plays important roles in regulating soil available nitrogen and plant biomass in alpine grassland ecosystems under climate changes, Agric. Ecosyst. Environ., 2019, vol. 279, pp. 187–193. Translated by the authors
ACKNOWLEDGMENTS
We are grateful to Holding Aqua LLC for providing the access to the production wells of YMWD.
Funding
This work was supported by the grant from the Russian Science Foundation no. 21-14-00333 (2021 year sampling, isolation and physiological characterization of pure cultures, hydrogeological analysis, sequencing, and genomic analysis, manuscript preparation), D.G.Z, M.I.P., A.A.K., A.Y.M., S.N.G. also acknowledge support from the Russian Ministry of Science and Higher Education (sampling and DNA extraction).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
The original online version of this article was revised: Due to a retrospective Open Access order.
Supplementary Information
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zavarzina, D.G., Prokofeva, M.I., Pikhtereva, V.A. et al. Deferrivibrio essentukiensis sp. nov., gen. nov., a Representative of Deferrivibrionaceae fam. nov., Isolated from the Subsurface Aquifer of Caucasian Mineral Drinking Waters. Microbiology 91, 143–159 (2022). https://doi.org/10.1134/S0026261722020114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722020114