Abstract
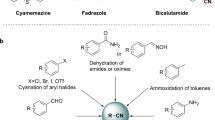
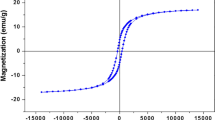
Nano-α-Fe2O3 prepared using acid pickling waste as precursor was found to be an effective catalyst for benzylation aniline with benzyl chloride. However, the formation of a green solution was observed during the solvent-free benzylation. A yellow-green single crystal as a byproduct was recovered from the green reaction mixture and characterized by X-ray single crystal diffraction, 1H NMR, FT-IR and elemental analysis. The results showed that this single crystal was a composite structural complex constructed by [(C6H6–CH2)2NH–C6H6]FeCl4 and [(C6H6–CH2)2NH–C6H6]Cl in equimolar ratio. The formation of the complex led to the deactivation and non-reusability of α-Fe2O3 catalyst for catalyzing solvent-free N-benzylation of aniline with benzyl chloride. The forming mechanism of the crystal was also discussed.







Similar content being viewed by others
REFERENCES
Ju, Y. and Varma, R.S., Green Chem., 2004, vol. 6, p. 219.
Dolle, R.E., Bourdonnec, B.L., Goodman, A.J., Morales, G.A., Thomas, C.J., and Zhang, W.J., Comb. Chem., 2009, vol. 11, p. 739.
Insaf, S.S. and Witiak, D.T., Synthesis, 1999, vol. 1999, p. 435.
Hamid, M.H.S.A., Allen, C.L., Lamb, G.W., Maxwell, A.C., Maytum, H.C., Watson, A.J.A., and Williams, J.M.J., J. Am. Chem. Soc., 2009, vol. 131, p. 1766.
Yin, M., He, S., Yu, Z., Wu, K., Wang, L., and Sun, C., Chin. J. Catal., 2013, vol. 34, p. 1534.
Artamkina, G.A., Ermolina, M.V., and Beletskaya, I.P., Mendeleev Commun., 2003, vol. 13, p. 158.
Fujita, K., Li, Z., Ozeki, N., and Yamaguchi, R., Tetrahedron Lett., 2003, vol. 44, p. 2687.
Xu, H. and Wolf, C., Chem. Commun., 2009, p. 1715.
Wang, D. and Ding, K., Chem. Commun., 2009, p. 1891.
Gupta, M., Paul, S., and Gupta, R., Chin. J. Catal., 2014, vol. 35, p. 444.
Xu, H. and Wolf, C., Chem. Commun., 2009, p. 3035.
Tang, H., Wang, H., Lu, B., Zhao, J., and Cai, Q., Mol. Catal., 2017, vol. 431, p. 27.
Li, X., You, X., Lu, B., Wu, X., Zhao, J., and Cai, Q., Ind. Eng. Chem. Res., 2014, vol. 53, p. 20085.
Funding
This research was funded by National Natural Science Foundation of China (grant no. 21 671 050).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Abbreviations: DPA, N-dibenzyl phenyl aminium chloride.
SUPPORTING INFORMATION
SUPPORTING INFORMATION
Rights and permissions
About this article
Cite this article
Ding, C., Wang, H., Lu, B. et al. Studies on Deactivation of Iron Oxide Catalyst in Solvent-Free N-Benzylation of Aniline with Benzyl Chloride. Kinet Catal 61, 768–774 (2020). https://doi.org/10.1134/S0023158420050031
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158420050031