Abstract
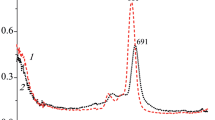
Electronic structures of four tetrafluoro derivatives of zinc(II) phthalocyanine with substituents at non-peripheral positions as well as their valence electronically excited singlet states are calculated by density functional theory methods. For the structural isomers under study intense electronic transitions to which characteristic Q and B absorption bands correspond are analyzed. The UV-Vis absorption spectrum of the considered compound is modeled using the calculated data for the relative thermodynamic stability of the isomers.




Similar content being viewed by others
REFERENCES
G. Guillaud, J. Simon, and J. P. Germain. Metallophthalocyanines: Gas sensors, resistors and field effect transistors. Coord. Chem. Rev., 1998, 178-180, 1433-1484. https://doi.org/10.1016/S0010-8545(98)00177-5
G. de , P. Vázquez, F. Agulló-López, and T. Torres. Role of structural factors in the nonlinear optical properties of phthalocyanines and related compounds. Chem. Rev., 2004, 104(9), 3723-3750. https://doi.org/10.1021/cr030206t
G. de , C. G. Claessens, and T. Torres. Phthalocyanines: old dyes, new materials. Putting color in nanotechnology. Chem. Commun., 2007, (20), 2000-2015. https://doi.org/10.1039/B614234F
S. Campidelli, B. Ballesteros, A. Filoramo, D. D. Díaz, G. de , T. Torres, G. M. A. Rahman, C. Ehli, D. Kiessling, F. Werner, V. Sgobba, D. M. Guldi, C. Cioffi, M. Prato, and J.-P. Bourgoin. Facile decoration of functionalized single-wall carbon nanotubes with phthalocyanines via “click chemistry”. J. Am. Chem. Soc., 2008, 130(34), 11503-11509. https://doi.org/10.1021/ja8033262
C. G. Claessens, U. Hahn, and T. Torres. Phthalocyanines: From outstanding electronic properties to emerging applications. Chem. Rec., 2008, 8(2), 75-97. https://doi.org/10.1002/tcr.20139
G. Bottari, G. de , D. M. Guldi, and T. Torres. Covalent and noncovalent phthalocyanine–carbon nanostructure systems: synthesis, photoinduced electron transfer, and application to molecular photovoltaics. Chem. Rev., 2010, 110(11), 6768-6816. https://doi.org/10.1021/cr900254z
M.-S. Liao and S. Scheiner. Electronic structure and bonding in metal phthalocyanines, metal = Fe, Co, Ni, Cu, Zn, Mg. J. Chem. Phys., 2001, 114(22), 9780-9791. https://doi.org/10.1063/1.1367374
M. Schwarze, W. Tress, B. Beyer, F. Gao, R. Scholz, C. Poelking, K. Ortstein, A. A. Günther, D. Kasemann, D. Andrienko, and K. Leo. Band structure engineering in organic semiconductors. Science, 2016, 352(6292), 1446. https://doi.org/10.1126/science.aaf0590
H. Lu and N. Kobayashi. Optically active porphyrin and phthalocyanine systems. Chem. Rev., 2016, 116(10), 6184-6261. https://doi.org/10.1021/acs.chemrev.5b00588
A. G. Martynov, E. A. Safonova, A. Yu. Tsivadze, and Y. G. Gorbunova. Functional molecular switches involving tetrapyrrolic macrocycles. Coord. Chem. Rev., 2019, 387, 325-347. https://doi.org/10.1016/j.ccr.2019.02.004
A. G. Martynov, J. Mack, A. K. May, T. Nyokong, Y. G. Gorbunova, and A. Y. Tsivadze. Methodological survey of simplified TD-DFT methods for fast and accurate interpretation of UV-Vis-NIR spectra of phthalocyanines. ACS Omega, 2019, 4(4), 7265-7284. https://doi.org/10.1021/acsomega.8b03500
Q. Zhou, Z.-F. Liu, T. J. Marks, and P. Darancet. Electronic structure of metallophthalocyanines, MPc (M = Fe, Co, Ni, Cu, Zn, Mg) and fluorinated MPc. J. Phys. Chem. A, 2021, 125(19), 4055-4061. https://doi.org/10.1021/acs.jpca.0c10766
T. Mayer, U. Weiler, C. Kelting, D. Schlettwein, S. Makarov, D. Wöhrle, O. Abdallah, M. Kunst, and W. Jaegermann. Silicon-organic pigment material hybrids for photovoltaic application. Sol. Energy Mater. Sol. Cells, 2007, 91(20), 1873-1886. https://doi.org/10.1016/j.solmat.2007.07.004
H. Jiang, P. Hu, J. Ye, Y. Li, H. Li, X. Zhang, R. Li, H. Dong, W. Hu, and C. Kloc. Molecular crystal engineering: tuning organic semiconductor from p-type to n-type by adjusting their substitutional symmetry. Adv. Mater., 2017, 29(10), 1605053. https://doi.org/10.1002/adma.201605053
D. Klyamer, D. Bonegardt, and T. Basova. Fluoro-substituted metal phthalocyanines for active layers of chemical sensors. Chemosensors, 2021, 9(6), 133. https://doi.org/10.3390/chemosensors9060133
D. Bonegardt, D. Klyamer, A. Sukhikh, P. Krasnov, P. Popovetskiy, and T. Basova. Fluorination vs. chlorination: effect on the sensor response of tetrasubstituted zinc phthalocyanine films to ammonia. Chemosensors, 2021, 9(6), 137. https://doi.org/10.3390/chemosensors9060137
D. Klyamer, A. Sukhikh, S. Gromilov, P. Krasnov, and T. Basova. Fluorinated metal phthalocyanines: interplay between fluorination degree, films orientation, and ammonia sensing properties. Sensors, 2018, 18(7), 2141. https://doi.org/10.3390/s18072141
A. S. Nizovtsev. Structural isomers and vibrational spectrum of tetrafluorosubstituted zinc phthalocyanine. J. Struct. Chem., 2022, 63(9), 1491-1495. https://doi.org/10.1134/S0022476622090104
A. D. Becke. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys., 1993, 98(7), 5648-5652. https://doi.org/10.1063/1.464913
C. Lee, W. Yang, and R. G. Parr. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B, 1988, 37(2), 785-789. https://doi.org/10.1103/PhysRevB.37.785
S. Grimme, J. Antony, S. Ehrlich, and H. Krieg. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys., 2010, 132(15), 154104. https://doi.org/10.1063/1.3382344
S. Grimme, S. Ehrlich, and L. Goerigk. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem., 2011, 32(7), 1456-1465. https://doi.org/10.1002/jcc.21759
F. Weigend and R. Ahlrichs. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys., 2005, 7(18), 3297-3305. https://doi.org/10.1039/B508541A
N. Mardirossian and M. Head-Gordon. ωB97M-V: A combinatorially optimized, range-separated hybrid, meta-GGA density functional with VV10 nonlocal correlation. J. Chem. Phys., 2016, 144(21), 214110. https://doi.org/10.1063/1.4952647
O. A. Vydrov and T. Van Voorhis. Nonlocal van der Waals density functional: The simpler the better. J. Chem. Phys., 2010, 133(24), 244103. https://doi.org/10.1063/1.3521275
W. Hujo and S. Grimme. Performance of the van der Waals density functional VV10 and (hybrid)GGA variants for thermochemistry and noncovalent interactions. J. Chem. Theory Comput., 2011, 7(12), 3866-3871. https://doi.org/10.1021/ct200644w
C. van Wüllen. Molecular density functional calculations in the regular relativistic approximation: Method, application to coinage metal diatomics, hydrides, fluorides and chlorides, and comparison with first-order relativistic calculations. J. Chem. Phys., 1998, 109(2), 392-399. https://doi.org/10.1063/1.476576
F. Neese, F. Wennmohs, A. Hansen, and U. Becker. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ′chain-of-spheres′ algorithm for the Hartree–Fock exchange. Chem. Phys., 2009, 356(1-3), 98-109. https://doi.org/10.1016/j.chemphys.2008.10.036
R. Izsák and F. Neese. An overlap fitted chain of spheres exchange method. J. Chem. Phys., 2011, 135(14), 144105. https://doi.org/10.1063/1.3646921
F. Weigend. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys., 2006, 8(9), 1057-1065. https://doi.org/10.1039/B515623H
T. Yanai, D. P. Tew, and N. C. Handy. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett., 2004, 393(1), 51-57. https://doi.org/10.1016/j.cplett.2004.06.011
L. Kronik, T. Stein, S. Refaely-Abramson, and R. Baer. Excitation gaps of finite-sized systems from optimally tuned range-separated hybrid functionals. J. Chem. Theory Comput., 2012, 8(5), 1515-1531. https://doi.org/10.1021/ct2009363
J. Autschbach and M. Srebro. Delocalization error and “functional tuning” in Kohn–Sham calculations of molecular properties. Acc. Chem. Res., 2014, 47(8), 2592-2602. https://doi.org/10.1021/ar500171t
T. Körzdörfer and J.-L. Brédas. Organic electronic materials: recent advances in the DFT description of the ground and excited states using tuned range-separated hybrid functionals. Acc. Chem. Res., 2014, 47(11), 3284-3291. https://doi.org/10.1021/ar500021t
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox. Gaussian09, Revision D.01. Wallingford, CT, USA: Gaussian, Inc., 2013.
F. Neese. Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci., 2018, 8(1), e1327. https://doi.org/10.1002/wcms.1327
R. L. Martin. Natural transition orbitals. J. Chem. Phys., 2003, 118(11), 4775-4777. https://doi.org/10.1063/1.1558471
Z. Liu, T. Lu, and Q. Chen. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon, 2020, 165, 461-467. https://doi.org/10.1016/j.carbon.2020.05.023
T. Lu and F. Chen. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem., 2012, 33(5), 580-592. https://doi.org/10.1002/jcc.22885
L. Edwards and M. Gouterman. Porphyrins: XV. Vapor absorption spectra and stability: Phthalocyanines. J. Mol. Spectrosc., 1970, 33(2), 292-310. https://doi.org/10.1016/0022-2852(70)90040-8
N. Kobayashi, H. Ogata, N. Nonaka, and E. A. Luk′yanets. Effect of peripheral substitution on the electronic absorption and fluorescence spectra of metal-free and zinc phthalocyanines. Chem. Eur. J., 2003, 9(20), 5123-5134. https://doi.org/10.1002/chem.200304834
J. Mack and N. Kobayashi. Low symmetry phthalocyanines and their analogues. Chem. Rev., 2011, 111(2), 281-321. https://doi.org/10.1021/cr9003049
V. N. Nemykin, R. G. Hadt, R. V. Belosludov, H. Mizuseki, and Y. Kawazoe. Influence of molecular geometry, exchange-correlation functional, and solvent effects in the modeling of vertical excitation energies in phthalocyanines using time-dependent density functional theory (TDDFT) and polarized continuum model TDDFT methods: Can modern computational chemistry methods explain experimental controversies? J. Phys. Chem. A, 2007, 111(50), 12901-12913. https://doi.org/10.1021/jp0759731
D. Bonegardt, D. Klyamer, P. Krasnov, A. Sukhikh, and T. Basova. Effect of the position of fluorine substituents in tetrasubstituted metal phthalocyanines on their vibrational spectra. J. Fluor. Chem., 2021, 246, 109780. https://doi.org/10.1016/j.jfluchem.2021.109780
Funding
The work was supported by the Russian Science Foundation (grant No. 21-73-00276).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 7, 112979.https://doi.org/10.26902/JSC_id112979
Supplementary material
Rights and permissions
About this article
Cite this article
Nizovtsev, A.S. Optical Spectrum of Tetrafluorosubstituted Zinc Phthalocyanine. J Struct Chem 64, 1228–1236 (2023). https://doi.org/10.1134/S0022476623070077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623070077