Abstract
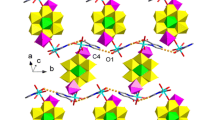
N,N,N′,N′-tetramethylethylenediamine is obtained by the reaction of ethylenediamine with formaldehyde and formic acid (the Eschweiler-Clarke reaction) and then alkylated with allyl chloride (or bromide) in a ratio of 1:1 or 1:2 to obtain N-allyl-N,N,N′,N′-tetramethylethylenediaminium and N,N′-diallyl-N,N,N′,N′-tetramethylethylenediaminium bromide respectively. [{C2H4N2(H+)(CH3)4(C3H5)}Cu4Cl6] (1) and [{C2H4N2(CH3)4(C3H5)2}0.5Cu2Cl1.67Br1.33] (2) π-complexes are obtained from alcohol solutions containing an ethylenediamine derivative and copper(II) chloride by ac-electrochemical synthesis on copper wire electrodes. An XRD study of the complexes is carried out. The crystals are monoclinic; 1: P21/n space group, a = 9.0081(6) Å, b = 12.5608(7) Å, c = 16.8610(10) Å, β = 102.061(3)°, V = 1865.7(2) Å3, Z = 4; 2: C2/c space group, a = 14.462(2) Å, b = 12.519(1) Å, c = 12.762(2) Å, β = 107.861(5)°, V = 2199.1(4) Å3, Z = 8. The structure of 1 consists of infinite copper halide networks with four crystallographically independent copper atoms, one of which coordinates the double bond of the allyl group of the ligand. The [C2H4N2(H)(CH3)4(C3H5)]2+ cations are attached above and below the plane of the network. The individual fragments are bonded via an extensive system of (N)H…Cl and (C)H…Cl hydrogen bonds. The structure of 2 contains a three-dimensional copper halide framework whose cavities contain the [C2H4N2(CH3)4(C3H5)2]2+ cations that are π-coordinated with copper(I) atoms. In both structures, the Cu(I) atom that coordinates the C=C bond has a trigonal-pyramidal coordination environment consisting of the double C=C bond of the corresponding ligand and three halogen atoms. The other Cu(I) atoms have a tetrahedral environment consisting solely of halogen atoms. The Cu-(C=C) distance is 1.958(1) Å, (1) and 1.974(1) Å (2).
Similar content being viewed by others
References
I. Bertini, P. Dapporto, D. Gatteschi, et al., J. Chem. Soc., Dalton Trans., No. 9, 1409 (1979).
J. R. Anacona, C. Gutierrez, and C. Rodriguez-Barbarin, Monatsh. Chem., 135, No. 7, 785 (2004).
R. D. Ball, D. Hall, C. E. F. Rickard, et al., J. Chem. Soc. A, 1435 (1967).
M. Pasquali, C. Floriani, and A. Gaetani-Manfredotti, Inorg. Chem., 19, No. 5, 1191 (1980).
A. Johansson, M. Hakansson, and S. Jagner, Chem. Eur. J., 11, No. 18, 5311 (2005).
E. D. Estes, W. E. Estes, W. E. Hatfield, et al., Inorg. Chem., 14, No. 1, 106 (1975).
M. R. Churchill, G. Davies, M. A. El-Sayed, et al., Inorg. Chem., 23, No. 6, 783 (1984).
M. M. Monchak, A. V. Pavlyuk, V. V. Kinzhibalo, et al., Koordinatts. Khim., 35, No. 6, 414 (2009).
M. Monchak, E. Goreshnik, and M. Mys’kiv, Visn. Lviv. Univ. Ser. Khim., No. 50, 36 (2009).
M. Monchak, A. V. Pavlyuk, V. V. Kinzhibalo, et al., Koordinats. Khim., 36, No. 3, 200 (2010).
K. Weygand and G. Hilgetag, Organisch-Chemische Experimentierkunst, Barth, Leipzig (1964).
B. M. Mykhalichko and M. G. Mys’kiv, Patent of Ukraine No. UA 25450A, Bull., No. 6 (1998).
CrystalClear: Rigaku Corporation, The Woodlands, Texas, USA (1999).
R. C. Clark and J. S. Reid, Acta Cryst., A51, No. 6, 887 (1995).
A. Altomare, G. Cascarano, C. Giacovazzo, et al., J. Appl. Cryst., 27, 435 (1994).
G. M. Sheldrick, SHELXS-97 and SHELXL-97, Program for the Solution and Refinement of Crystal Structures, Univ. Göttingen, Germany (1997).
G. R. Desiraju and T. Steiner, Weak Hydrogen Bond in Structural Chemistry and Biology, Oxford University Press, Oxford (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2012 by M. M. Monchak, E. A. Goreshnik, and M. G. Mys’kiv
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 53, No. 1, pp. 124–129, January–February, 2012.
Rights and permissions
About this article
Cite this article
Monchak, M.M., Goreshnik, E.A. & Mys’kiv, M.G. Architecture of framework copper(I) halide π-complexes with N-allyl-N,N,N′,N′-tetramethyl-ethylenediaminium and N,N′-diallyl-N,N,N′,N′-tetramethylethylenediaminium: Synthesis and crystal structure of [{C2H4N2(H+)×(CH3)4(C3H5)} Cu4Cl6] and [{C2H4N2(CH3)4(C3H5)2}0.5Cu2Cl1.67Br1.33]. J Struct Chem 53, 119–124 (2012). https://doi.org/10.1134/S0022476612010155
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476612010155