Abstract—
A working process is proposed for an HF(DF) laser, in which evacuation of the reactor is completely excluded. The workflow consists of the following stages: (1) displacement of the reaction products, which are formed after initiation of the working mixture, by an inert gas and (2) subsequent displacement of the inert gas by the working mixture. A design solution is proposed for the system of gas puffing into the reactor. Gas is supplied through an annular slot at one of the reactor ends, which makes it possible to minimize the consumption of inert gas used for displacing the reaction products after initiation of the working mixture. The amount of residual HF(DF) gas in the reactor is incapable of affecting the generation energy in the next cycle. It has been experimentally shown that the displacement technology does not lower the lasing energy in comparison with the pumping technology, but significantly reduces the duration of the HF(DF)-laser operating cycle by approximately two orders of magnitude).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
The working mixture of a chemical HF(DF) laser, in addition to the main reagents F2 and H2(D2), contains O2, which is needed for its stabilization, and an inert diluent gas (usually He). The optimum mixture contains F2 and H2(D2) at a ratio of 3 : 1 and O2 and F2 at a ratio of 1 : 5. As a result, the working mixture has a composition of 0.15F2 + 0.03O2 + 0.05H2(D2) + 0.77He. The standard operating cycle of a pulsed chemical HF(DF) laser includes the following stages: (1) preparation of the working mixture in a pre-evacuated reactor and its initiation and (2) pumping out of the reaction products through a filter that absorbs corrosive gases, i.e., HF(DF) and residual F2. The advantage of such a cycle is the operability at an arbitrary pressure of the working mixture in the reactor, while its disadvantage is the long time it takes to fill the reactor with the working mixture, since one needs to take various precautions due to the branched-chain mechanism of the reaction between F2 and H2(D2) [1]. The other disadvantage is the prolonged (approximately 10 min) cleaning of the reactor by pumping out the products formed after the initiation of the working mixture. As a result, the time interval between two successive pulses is 10 min or more.
Our investigations aimed at solving the problem of fast and safe preparation of hydrogen fluoride mixtures were described in [1, 2]. The technology of fast (in a few seconds) preparation of working mixtures with the simultaneous filling of the reactor with reagents was substantiated in [1]. The reaction of F2 with H2(D2) proceeds with energetic branching, as a result of which preparation of mixtures can be accompanied by spontaneous combustion. To eliminate spontaneous combustion of the injected mixture in the reactor in the initial period, we propose to pre-fill the reactor with a certain amount of inert gas. The minimum pressure of the inert gas at which the delivered mixture does not ignite depends on the type of inert gas, the mixture composition, the blow rate, and other factors; this requires experimental determination. It was proposed that a mixer used for fast and safe preparation of mixtures consist of a system of capillaries tightly packed into a cylindrical channel. A mixture containing H2(D2) is fed through the capillaries, and a mixture containing F2 is fed through the spaces between the capillaries. The design of a compact capillary mixer providing efficient mixing of gases with a total capacity of up to 2 L atm/s was described in [2].
In this work, we propose to exclude one more long stage from the working cycle, namely, the pumping out of reaction products. Displacement of reaction products from the reactor with an inert gas should be used instead. This basically allows us to significantly reduce the cycle time of a chemical laser. This technology implies that the puffed working mixture displaces the gas remaining in the reactor after the previous cycle; after the mixture is initiated the inert gas displaces the products formed after the explosion. If the products are displaced into the ambient atmosphere (through a filter that absorbs corrosive gases) the pressure of displacing gases must not be lower than the atmospheric pressure.
2 EXPERIMENTAL
Two variants of gas puffing into the reactor were used in the experiments with the gas displacement: (1) puffing through a 1-cm-diameter channel located at an angle of 45° with the reactor axis and (2) puffing through an annular (along the reactor diameter) slot 0.35 mm in width.
The channel outlet, as well as the annular slot, was located near the end of the reactor. The cross-sectional areas of the channel and the annular slot were approximately the same: 0.79 and 0.72 cm2, respectively. In both variants of gas puffing (using the channel or the slot), the gas was displaced from the reactor through a 1-cm-diameter side hole located at the opposite end.
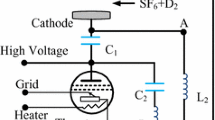
The variant with the puffing through the annular slot is schematically shown in Fig. 1. In this embodiment, the working mixture is initially fed through the inlet tube into the distributional toroidal cavity, from which it then flows into the reactor through the annular slot.
2.1 Displacement of an Inert Gas from the Reactor with a Working Mixture
A chemical DF laser with a Teflon reactor with a length of 120 cm and a volume of 4 L we used in the experiments. The mixture was initiated by flash lamps with a total stored energy of 440 J. The laser radiation energy was 20 J. A simplified diagram of the puffing device is shown in Fig. 2. It includes three independent pipelines for supplying mixtures of 0.3F2 + 0.06O2 + 0.64He, 0.1D2 + 0.9He, and pure He, respectively. The device contains a mixer M, electromagnetic valves EV1–EV4, and a system for controlling the flow rates of puffed gases (not shown in the figure) [2]. The working mixture is formed as a combination of mixtures 0.3F2 + 0.06O2 + 0.64He and 0.1H2(D2) + 0.9He at their equal flow rates. The helium pipeline is used to displace products from the reactor. The electromagnetic valves are used to quickly supply and shut the flows of the respective gases. A mixer with a total throughput of 2 L atm/s was used to prepare the working mixture [2]. Upon simultaneous opening of the electromagnetic valves EV1, EV2, and EV4, the working mixture formed in the mixer displaces the helium, which was preliminarily puffed into the evacuated reactor, through the EV4 valve. During the displacement, the pressure in the reactor remains unchanged at a level of 1 atm. The duration of displacement varies from 2 s (one times displacement) to 3 s (1.5 times displacement) while maintaining the volumetric flow rate at a level of 2 L atm/s. Hereinafter, the displacement ratio is understood as the ratio of the used volume of the displacement gas to the reactor volume.
The lasing was compared in the experiments with mixtures prepared using the displacement technology and mixtures prepared using the standard technology. According to this technology, the evacuated reactor is preliminarily filled with helium to a pressure of 0.1 atm; the working mixture is then puffed at a rate of 2 L atm/s to a total pressure of 1 atm. As noted, the preliminary filling of the reactor with helium prevents spontaneous ignition of the working mixture at the start of filling, while the pressure in the reactor is low [1]. The preliminary puffed helium is then displaced as the mixture is injected into the additional volume connected to the reactor. If the additional volume is ν0, the laser volume is ν, and P0 and P are the initial and final gas pressures, respectively, ν0/ν = P0/(P – P0). At ν = 4 L, P0 = 0.1 atm, and P = 1 atm, the additional volume is ν0 ~ 0.44 L.
The lasing density in joules per square centimeter was determined in the displacement experiments as a function of the ratio of helium displacement from the reactor with a mixture of 0.15F2 + 0.03O2 + 0.05D2 + 0.77He from the mixer. the design features of the devices for gas puffing into the reactor were taken into account. The results of the experiments performed using the standard and displacement technologies are shown below (each result was averaged over 3–5 experiments):
– standard technology for puffing the working mixture | 0.46 J/cm2 |
– one times displacement of helium with a mixture of 0.15F2 + 0.03O2 + 0.05D2 + 0.77He; mixture puffing through the channel | 0.53 J/cm2 |
– 1.5 times displacement of helium with a mixture 0.15F2 + 0.03O2 + 0.05D2 + 0.77He; mixture puffing through the channel | 0.65 J/cm2 |
– one times displacement of helium with a mixture of 0.15F2 + 0.03O2 + 0.05D2 + 0.77He; mixture puffing through the annular slot | 0.59 J/cm2 |
Analysis of the data shows that the lasing energy is generally higher when the displacement technology is used. An increase in the frequency of flushing the reactor with a working mixture and the puffing of the mixture through the annular slot also facilitate an increase in the lasing energy. We note that all this applies equally to a chemical HF laser.
2.2 Displacement of Reaction Products from the Reactor with an Inert Gas
The explosion products of the standard working mixture of 0.15F2 + 0.03O2 + 0.05D2 + 0.77He contain 10% DF. The problems that arise when the displacement technology is used to remove products are much more complex, since even a small amount of DF remaining after flushing the reactor with an inert gas can effectively reduce the lasing in the next cycle by introducing additional losses due to the absorption of laser radiation by DF molecules.
The efficiency of DF displacement was determined from the value of the lasing energy achieved in the experiments, depending on the product-displacement ratio and the method for injecting the displacing gas into the reactor.
The lasing in the experiments was compared to the lasing in the “standard” experiment. He was puffed into the evacuated reactor to a pressure of 1 atm and then was displaced with a working mixture. The working mixture was initiated and the lasing energy was measured. This experiment was considered standard. After it was carried out the He products were displaced. He with a possible DF impurity was then displaced with a new portion of the working mixture, the working mixture was initiated, and the lasing energy was measured and compared to the lasing in the previous experiment. The results of the experiments are presented below.
Displacement of products when He was supplied through the channel, J/cm2:
–standard experiment | 0.56 |
–3 times displacement of products with helium | 0 |
–10 times displacement of products with helium | 0 |
Displacement of products when He was supplied through the annular slot, J/cm2:
– standard experiment | 0.54 |
–1.4 times displacement of products with helium | 0.48 |
–2.8 times displacement of products with helium | 0.49 |
–4.2 times displacement of products with helium | 0.58 |
According to the data obtained, even a tenfold displacement of products when He was fed through the channel did not lead to lasing in the subsequent experiment. However, the use of the annular slot has solved the problem. In this case, the required displacement ratio was not high: 1.4 times displacement was quite enough.
The explanation of these results is as follows. The inlet channel and the reactor have markedly different diameters, so their coupling leads to a sudden expansion of the flow. A jet flow with a free boundary expanding in the direction of the jet motion occurs at the entrance to the reactor. The speed of the jet flowing out of the channel is about 25 m/s. Upon reaching the wall, the jet is successively reflected from it until the width of the jet equals the reactor diameter. The gas flow occurs thereafter with a fixed outer boundary. A complex vortex motion is established between the wall and the jet boundary in the section where the expanded jet reaches the reactor size. Stagnant zones with turbulent mass exchange between the helium jet and the surrounding gas (the products of the mixture explosion in the previous experiment) are formed. A significant amount of stationary gas from the reactor can be mixed with the helium flow in stagnant zones. As a result, the products are displaced not by the incoming helium, but by a mixture of helium with the explosion products, which can lead to an increase in the concentration of the residual DF.
When the annular slot is used, the helium flowing out of it creates a gas “curtain.” While moving towards the other end of the reactor, this curtain creates a type of inert-gas plug, which displaces the products like a piston. When the reactor-filling time is 2 s, as was the case in our experiments, the diffusion smearing of the plug front is small and the plug configuration is retained over the total reactor-filling time. This explains the high efficiency of product displacement when the annular slot is used.
3 CONCLUSIONS
These data demonstrate the feasibility of the working process of a HF(DF) laser, in which the reactor evacuation process is completely eliminated without a loss in the lasing energy. The process consists of two stages: (1) displacement of reaction products, which were formed upon the initiation of the working mixture, by an inert gas and (2) subsequent displacement of the inert gas by the working mixture. The duration of each stage depends on the volumetric rate of gas puffing into the reactor and the reactor volume. A working-cycle time of approximately 5 s was achieved at the laser device with a 120-cm-long 4-L-volume reactor and a mixer with a throughput of 2 L atm/s and a laser-pulse energy of 20 J. This value is less by approximately two orders of magnitude than the cycle time with the pumping. If the gas is displaced into the atmosphere, the working pressure in the reactor must be atmospheric or higher.
REFERENCES
Vasil’ev, G.K., Makarov, E.F., and Chernyshev, Yu.A., Fiz. Goreniya Vzryva, 2003, vol. 39, no. 3, p. 9.
Agroskin, V.Ya., Bravy, B.G., Vasiliev, G.K., Guriev, V.I., Kashtanov, S.A., Makarov, E.F., Sotnichenko, S.A., and Chernyshev, Yu.A., Instrum. Exp. Tech., 2019, vol. 62, no. 1, pp. 101–104. https://doi.org/10.1134/S0020441218060015
Funding
This work was supported by the state order, no. AAAA-A19-119070790003-7.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by N. Goryacheva
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agroskin, V.Y., Bravy, B.G., Vasiliev, G.K. et al. An Effective Pulsed-Periodic Chemical HF(DF) Laser. Instrum Exp Tech 64, 830–833 (2021). https://doi.org/10.1134/S0020441221060014
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020441221060014