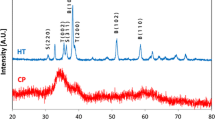
Abstract—
We have studied the process underlying the preparation of molybdenum–chromium alloy powders via the magnesium vapor reduction of the Cr2(MoO4)3 chromium molybdate in the temperature range from 700 to 800°C at residual argon pressures in the reactor from 5 to 15 kPa and obtained powders of Mo0.3Cr0.7 alloy, a mixture of Mo0.3Cr0.7 and Mo0.7Cr0.3 alloys, and alloys of a continuous series of MoхCr1 – х (0 < x < 1) solid solutions. The powders range in specific surface area from 33 to 48 m2/g and have a mesoporous structure.








Similar content being viewed by others
REFERENCES
Tablitsy fizicheskikh velichin (Tables of Physical Quantities), Kikoin, I.K., Ed., Moscow: Atomizdat, 1976.
Xiao, M., Li, F., Xie, H., and Wang, Y., Characterization of strengthening mechanism and hot deformation behavior of powder metallurgy molybdenum, Mater. Des., 2012, vol. 34, no. 2, pp. 112–119.
Shields, J.A., Applications of Molybdenum Metal and Its Alloys, London: IMOA, 2013.
Jones, E.S., Mosher, J.F., Speiser, R., and Spretnak, J.W., The oxidation of molybdenum, Corrosion, 1958, vol. 14, no. 1, pp. 20–26.https://doi.org/10.5006/0010-9312-14.1.20
Zaitsev, A.A., Korotkov, N.A., and Lazarev, E.M., Oxidation of molybdenum and molybdenum–tungsten alloys, Metalloved. Term. Obrab. Met., 1976, no. 10, pp. 34–38. https://doi.org/10.1007/bf00705195
Dubinin, G.N. and Mulyakaev, L.M., Thermal stability of chromated molybdenum, Metalloved. Term. Obrab. Met., 1969, no. 11, pp. 35–39.https://doi.org/10.1007/BF00655521
Lee, D.-B. and Simkovich, G., Oxidation of molybdenum–chromium–palladium alloys, Oxid. Met., 1990, vol. 34, no. 12, pp. 13–22.
Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys, Landolt-Börnstein, New Series IV, vol. 5d, Madelung, O., Ed., Berlin: Springer, 1994.
Barin, I. and Platzki, G., Thermochemical Data of Pure Substances, Weinheim: VCH, 1995.
Odusote, Y.A. and Popoola, A.I., Thermodynamic and surface properties of Cr–X, (X = Mo, Fe) liquid alloys, Am. J. Condens. Matter Phys., 2017, vol. 7, no. 10, pp. 57–66.https://doi.org/10.5923/j.ajcmp.20170703.01
Hahn, J.D., Wu, F., and Bellon, P., Cr–Mo solid solutions forced by high-energy ball milling, Metall. Mater. Trans. A, 2004, vol. 35, no. 10, pp. 1105–1111.https://doi.org/10.1007/s11661-004-1013-8
Sun, C.-F., Xi, S.-Q., Zhang, Y., et al., Thermodynamic characteristic and phase evolution in immiscible Cr–Mo binary alloys, Acta Metall. Sin., 2015, vol. 28, no. 8, pp. 1074–1081.https://doi.org/10.1007/s40195-015-0297-6
Sun, C.F., Xi, S.Q., Zhang, Y., et al., Synthesising amorphous Cr–Mo alloy via mechanical alloying of immiscible Cr and Mo elements, Mater. Res. Innovations, 2015, vol. 19, pp. S1-308–S1-311.https://doi.org/10.1179/1432891715Z.0000000001493
Sun, C., Hai, X., Xi, S., et al., New insights of solid-state alloying and amorphous–nanocrystalline cyclic phase transitions during Cr–40 wt. % Mo powder milling, J. Alloys Compd., 2018, vol. 731, no. 1, pp. 667–677.https://doi.org/10.1016/j.jallcom.2017.10.083
Edigaryan, A.A. and Polukarov, Yu.M., Electrodeposition of chromium and its alloys from Cr(III) sulfate solutions, Gal’vanotekh. Obrab. Poverkhn., 2001, vol. 9, no. 3, pp. 17–24.
Kuznetsov, V.V. and Matveev, D.V., Electrodeposition of chromium–molybdenum alloy from electrolyte based on chromium(III) sulfate, Russ. J. Electrochem., 2008, vol. 44, no. 6, pp. 740–744.https://doi.org/10.1134/S1023193508060153
Kolosov, V.N., Miroshnichenko, M.N., and Prokhorova, T.Yu., Preparation of Mo–W alloy powders via magnesium vapor reduction of complex oxide compounds, Tr. Kol’skogo Nauchn. Tsentra Ross. Akad. Nauk, 2018, issue 9, part 1, no. 1, pp. 285–289.https://doi.org/10.25702/KSC.2307-5252.2018.9.1.285-289
Kolosov, V.N., Miroshnichenko, M.N., and Prokhorova, T.Yu., Magnesium vapor reduction of complex double compounds of molybdenum with tungsten, J. Phys. Conf. Ser., 2019, vol. 1347, paper 012128.https://doi.org/10.1088/1742-6596/1347/1/012128
Wu, M.-Y., Wang, L., Jia, Y., et al., Theoretical study of hydration in Y2Mo3O12: effects on structure and negative thermal expansion, AIP Adv., 2015, vol. 5, paper 027126.https://doi.org/10.1063/1.4913361
Marinkovic, B.A., Jardim, P.M., de Avillez, R.R., and Rizzo, F., Negative thermal expansion in Y2Mo3O12, Solid State Sci., 2015, vol. 7, no. 11, pp. 1377–1383.https://doi.org/10.1016/j.solidstatesciences.2005.08.012
Tyagi, A.K., Achary, S.N., and Mathews, M.D., Phase transition and negative thermal expansion in A2(MoO4)3 system (A = Fe3+, Cr3+ and Al3+), J. Alloys Compd., 2002, vol. 339, nos. 1–2, pp. 1377–1383.
Yadagiri, M., Ramakrishna, S., Ravi, G., et al., Preparation, characterization and photocatalytic studies of Cr2(MoO4)3 and nitrogen-doped Cr2(MoO4)3, Chem. Chem. Technol., 2015, vol. 9, no. 4, pp. 391–399.https://doi.org/10.23939/chcht09.04.391
Oudghiri-Hassani, H., Synthesis, characterization and application of chromium molybdate for oxidation of methylene blue dye, J. Mater. Environ. Sci., 2018, vol. 9, no. 3, pp. 1051–1057.
Forzatti, P., Mari, C.M., and Villa, P., Defect structure and transport properties of Cr2(MoO4)3 and Al2(MoO4)3, Mater. Res. Bull., 1987, vol. 22, no. 12, pp. 1593–1602.https://doi.org/10.1016/0025-5408(87)90001-8
Tabero, P., Synthesis of Cr2(MoO4)3, React. Kinet. Catal. Lett., 1999, vol. 67, no. 1, pp. 137–141.https://doi.org/10.1007/bf02475839
Cullity, B.D. and Stock, S.R., Elements of X-Ray Diffraction, Englewood Cliffs: Prentice-Hall, 2001, 3rd ed.
Kolosov, V.N. and Orlov, V.M., Electronically mediated reactions in metal thermal reduction of molybdenum and tungsten oxide compounds, Dokl. Phys. Chem., 2019, vol. 484, no. 2, pp. 28–31.https://doi.org/10.1134/S0012501619020027
Kolosov, V.N., Orlov, V.M., and Miroshnichenko, M.N., Calcium vapor reduction of group V and VI metal oxide compounds, Inorg. Mater., 2020, vol. 56, no. 1, pp. 35–41.https://doi.org/10.1134/S0020168520010069
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kolosov, V.N., Miroshnichenko, M.N. & Prokhorova, T.Y. Magnesiothermic Preparation of Molybdenum–Chromium Alloy Powders. Inorg Mater 58, 33–39 (2022). https://doi.org/10.1134/S0020168522010071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168522010071