Abstract
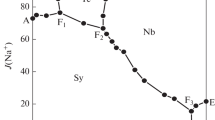
According to the compositions of the underground brine resources in the west of Sichuan Basin, solubilities of the ternary systems NaBr–Na2SO4–H2O and KBr–K2SO4–H2O were investigated by isothermal method at 348 K. The equilibrium solid phases, solubilities of salts, and densities of the solutions were determined. On the basis of the experimental data, the phase diagrams and the density-composition diagrams were plotted. In the two ternary systems, the phase diagrams consist of two univariant curves, one invariant point and two crystallization fields. Neither solid solution nor double salts were found. The equilibrium solid phases in the ternary system NaBr–Na2SO4–H2O are NaBr and Na2SO4, and those in the ternary system KBr–K2SO4–H2O are KBr and K2SO4. Using the solubilities data of the two ternary subsystems at 348 K, mixing ion-interaction parameters of Pitzer’s equation θxxx, Ψxxx and Ψxxx were fitted by multiple linear regression method. Based on the chemical model of Pitzer’s electrolyte solution theory, the solubilities of phase equilibria in the two ternary systems NaBr–Na2SO4–H2O and KBr–K2SO4–H2O were calculated with corresponding parameters. The calculation diagrams were plotted. The results showed that the calculated values have a good agreement with experimental data.
Similar content being viewed by others
References
C. Christov, “An isopiestic study of aqueous NaBr and KBr at 50°C: Chemical equilibrium model of solution behavior and solubility in the NaBr–H2O, KBr–H2O and Na–K–Br–H2O systems to high concentration and temperature,” Geochim. Cosmochim. Acta 71, 3557–3569 (2007).
C. Christov, “Isopiestic investigation of the osmotic coefficients of MgBr2(aq) and study of bromide salts solubility in the (m1KBr + m2KBr2)(aq) system at T = 323.15 K. Thermodynamic model of solution behaviour and (solid + liquid) equilibria in the MgBr2(aq), and (m1KBr + m2KBr2)(aq) systems to high concentration and temperature,” J. Chem. Thermodynam. 43, 344–353 (2011).
C. Christov, “Isopiestic investigation of the osmotic coefficients of aqueous CaBr2 and study of bromide salt solubility in the NaBr–CaBr2–H2O system at 50°C: Thermodynamic model of solution behavior and solid–liquid equilibria in the CaBr2–H2O, and NaBr–CaBr2–H2O systems to high concentration and temperature,” CALPHAD 35, 42–53 (2011).
C. Christov, “Study of bromide salts solubility in the (m1NaBr + m2KBr2)(aq) system at T = 323.15 K, Thermodynamic model of solution behavior and solid-liquid equilibria in the (Na + K + Mg + Br + H2O) system to high concentration and temperature,” J. Chem. Thermodynam. 47, 335–340 (2012).
C. Christov, “Study of bromide salts solubility in the (m1KBr + m2CaBr2)(aq) system at T = 323.15 K. Thermodynamic model of solution behaviour and (solid + liquid) equilibria in the ternaries (m1KBr + m2CaBr2)(aq), and (m1MgBr2 + m2CaBr2)(aq), and in the quinary (Na + K + Mg + Ca + Br + H2O) systems to high concentration and temperature,” J. Chem. Thermodynam. 55, 7–22 (2012).
C. Christov, and N. Moller, “A chemical equilibrium model of solution behavior and solubility in the H–Na–K–Ca–OH–Cl–HSO4–SO4–H2O system to high concentration and temperature,” Geochim. Cosmochim. Acta. 68, 3717–3739 (2004).
R. Z. Cui, S. H. Sang, and Y. X. Hu, “Solid–liquid equilibria in the quaternary systems KCl–KBr–K2B4O7–H2O and KCl–KBr–K2SO4–H2O at 373 K,” J. Chem. Eng. Data. 58, 477–481 (2013).
R. Z. Cui, S. H. Sang, Y. X. Hu, and J. W. Hu, “Phase equilibria in the ternary systems KBr–K2B4O7–H2O and KCl–K2B4O7–H2O at 373 K,” Acta Geol. Sinica 87 (6), 1668–1673 (2014).
R. Z. Cui, S. H. Sang, T. Li, and Y. G. Zhang, “Phase equilibria in the quinary system KCl–KBr–K2SO4–K2B4O7–H2O at 323 K and 348 K,” J. Chem. Ind. Eng. 64 (3), 827–833 (2013) [in Chinese].
J. P. Greenberg, and N. Moller, “The Prediction of mineral solubilities in natural water: a chemical equilibrium model for the Na–K–Ca–Cl–SO4–H2O system to highe concentration from 0 to 250°C,” Geochim. Cosmochim. Acta 53, 2503–2518 (1989).
C. E. Harvie, H. P. Eugster, and J. H. Weare, “Mineral equilibria in the six-component seawater system, Na–K–Mg–Ca–SO4–Cl–H2O at 25°C. II: Compositions of the saturated solutions,” Geochim. Cosmochim. Acta 46, 1603–1618 (1982).
C. E. Harvie, N. Moller, and J. H. Weare, “The prediction of mineral solubilities in natural waters: the Na–K–Mg–Ca–H–Cl–SO4–OH–HCO3–CO3–CO2–H2O system from zero to high concentration at 25°C,” Geochim. Cosmochim. Acta 48, 723–751 (1984).
C. E. Harvie, and J. H. Weare, “The prediction of mineral solubilities in natural waters: the Na–K–Mg–Ca–Cl–SO4–H2O system from zero to high concentrationat 25°C,” Geochim. Cosmochim. Acta 44, 981–997 (1980).
H. M. Hu, S. H. Sang, Y. G. Zhang, and J. J. Zhang, “Theoretical calculation of phase equilibrium for quaternary system study K+//Cl–, SO42−, B4O72−–H2O at 298 K,” J. Salt Chem. Ind. 41, 12–17 (2012).
Y. T. Lin, “Study on sustainable development of potassium boron iodine and bromine in brine of Sichuan basin,” J. Salt Lake Res. 9, 56–60 (2001).
Y. L. Lin, and S. X. Cao, “The utilization prospects of potassium-enriched and boron-enriched bittern in the west of the Sichuan basin,” Conserv. Util. Miner. Resour. 8 (1), 41–43 (1998).
Y. T. Lin, and S. L. Chen, “Exploitation and development prospect of underground brine in Sichuan Basin,” J. Salt Lake Res. 16, 1–7 (2008).
N. Moller, “The prediction of mineral solubilities in natural water: a chemieal model for the Na–Ca–Cl–SO4–H2O system to high temperatures and concentrations,” Geochim. Cosmochim. Acta. 52, 821–837 (1988).
S. H. Sang, T. Li, and R. Z. Cui, “A Study on Phase Equilibria in the Ternary Salt-water System KBr–K2B4O7–H2O at 348 K,” J. Salt Lake Res. 12, 29–32 (2013).
S. H. Sang, M. L. Sun, H. Li, X. Zhang, and K. J. Zhang, “A Study on Equilibria of the Quaternary System Na+,K+//Br–, SO42−–H2O at 323 K,” Chin. J. Inorg. Chem. 27, 845–849 (2011).
S. H. Sang, H. A. Yin, S. J. Ni, and C. J. Zhang, “A study on equilibrium solubilities and properties of solutions in the ternary system K2B4O7–KBr–H2O at 298 K,” J. Chengdu Univ. Tech. (Science & Technology Edition) (China) 33, 414–416 (2006).
P. S. Song, “Comprehensive utilization of salt lake and related resources,” J. Salt Lake Res. 8 (2), 33–57 (2000).
M. L. Sun, Sang, S. H. H. Li, and X. Y. Zhao, “Study on the phase equilibria in ternary system Na–Br–SO4–H2O at 323 K,” Chem. Eng. (China), 38(7), 67–70 (2010).
D. Wang, S. H. Sang, X. X. Zeng, and H. Y. Ning, “The phase equilibria of quaternary system KCl–KBr–K2SO4–H2O at 323 K,” Petrochem. Technol. 40, 285–288 (2011).
Z. L. Zhang, S. H. Sang, M. Li, and C. H. Hou, “Study on phase equilibrium of quaternary system K2B4O7–K2SO4–KCl–H2O at 298 K,” Chem. Eng(China). 37, 45–48 (2009).
K. J. Zhang, S. H. Sang, T. Li, and R. Z. Cui, “Liquid–solid equilibria in the quaternary system KCl–KBr–K2SO4–H2O at 348 K,” J. Chem. Eng. Data. 58, 115–117 (2013).
X. X. Zeng, S. H. Sang, D. Wang, and J. J. Zhang, “The theoretical calculations of phase equilibrium in the interactive quaternary system Na+,K+//Br–, SO42−–H2O at 323 K,” Chem. Eng. (China). 40, 32–35(2012).
X. Y. Zhao, S. H. Sang, and M. L. Sun, “Phase equilibrium of the ternary system K2B4O7–KBr–H2O at 323 K,” J. Salt Lake Res. 19, 35–39 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Sang, SH., Cui, RZ., Zhang, XP. et al. Measurements and calculations of solid-liquid equilibria in the ternary systems NaBr–Na2SO4–H2O and KBr–K2SO4–H2O at 348 K. Geochem. Int. 55, 1131–1139 (2017). https://doi.org/10.1134/S0016702917120047
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702917120047