Abstract
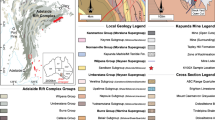
Data on the decommissioned Degtyarka Cu sulfide deposit, Urals, confirm the hypothesis that the flooding of abandoned mine workings is associated with the synthesis of secondary sulfates. Numerical simulation of hydrogeochemical processes in the rock—water system imitating the flooding of an underground void makes it possible to evaluate the conditions under which kirovite (Fe,Mg)SO4 · 7H2O and melanterite are formed at the oxidation of ore sulfides. Secondary sulfates are formed when the redox potential of the system is transformed from reducing to oxidizing within the stability field of Fe(II) species. The Fe/Mg ratio of the kirovite (Fe,Mg)SO4 · 7H2O is controlled first of all by the percentage of sulfides in the rock—water system, the rock/water ratio, the openness of the system with respect to atmospheric gases, and the temperature.
Similar content being viewed by others
References
L. K. Yakhontova and V. P. Zvereva, Minerals of Supergene Zone (Dal’nauka, Vladivostok, 2007) [in Russian].
E. V. Belogub, E. P. Shcherbakova, and N. K. Nikandrova, Sulfates in the Urals (Nauka, Moscow, 2007) [in Russian].
S. N. Elokhina and O. A. Silina, “Main tendencies in the chemical transformation of mining waters during flooding of copper mines in the Urals,” in Evolution of Scientific Ideas of A.M. Ovchinnkov in Hydrogeology, (MGGRU, Moscow, 2005), pp. 133–141 [in Russian].
A. S. Vershinin, O. N. Gryaznov, and V. I. Chesnokov, Theoretical Principles of Geochemical Methods of Mineral Exploration: A Textbook (UGGGA, Yekaterinburg, 2000) [in Russian].
Yu. V. Shvarov, “HCh: new potentialities for the thermodynamic simulation of geochemical systems offered by Windows,” Geochem. Int. 46 (8), 834–839 (2008).
Borisov, M.V. and Shvarov, Yu.V., Thermodynamics of Geochemical Processes (Mosk. Gos. Univ., Moscow, 1992) [in Russian].
K.-D. Grevel and J. Majzan, “Internally consistent thermodynamic data for metal divalent sulphate hydrates,” Chem. Geol. 286 (3–4), 301–306 (2011).
G. B. Naumov, B. N. Ryzhenko, and I. L. Khodakovsky, Handbook of Thermodynamic Data Ed. by A. I. Tugarinov (Atomizdat, Moscow, 1971).
S. R. Krainov, B. N. Ryzhenko, and V. M. Shvets, Geochemistry of Groundwaters. Theoretical, Applied, and Ecological Aspects (Nauka, Moscow, 2004) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.N. Elokhina, B.N. Ryzhenko, 2014, published in Geokhimiya, 2014, Vol. 52, No. 2, pp. 178–192.
Rights and permissions
About this article
Cite this article
Elokhina, S.N., Ryzhenko, B.N. Secondary mineral-forming processes in natural—anthropogenic hydrogeological systems at sulfide deposits. Simulation of the origin of the phase (Fe,Mg)SO4 · 7H2O in the course of sulfide oxidation at the Degtyarka copper sulfide deposit. Geochem. Int. 52, 162–177 (2014). https://doi.org/10.1134/S0016702914020050
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702914020050