Abstract
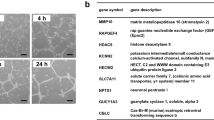
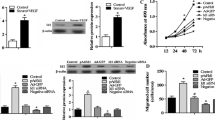
Cadherin is an epidermal growth factor and laminin-G seven-pass G-type receptor 1 (CELSR1) is a key component of the noncanonical Wnt/planar cell polarity (PCP) pathway that critically regulates endothelial cell proliferation and angiogenesis. In this study, we examined the biological significance of CELSR1 in endothelial cell migration and angiogenesis. For this, we applied both gain-of-function and loss-of-function approaches. To increase the endogenous expression of CELSR1, we used the transcription activator-like effector (TALE) technology and constructed an artificial TALE-VP64 activator. To knock down the expression of CELSR1, we generated lentivirus containing short hairpin RNA sequences targeting different regions of CELSR1 mRNA. Following up- or down-regulation of CELSR1 in human aortic endothelial cells (HAEC), we assessed in vitro cell proliferation by MTT assay, migration by scratch and transwell migration assays, and angiogenesis by tube formation analysis. We found that CELSR1 was endogenously expressed in human umbilical vein endothelial cells (HUVEC) and HAEC. When focusing on HAEC, we found that upregulating CELSR1 expression significantly promoted cell growth, while knocking down CELSR1 inhibited the growth (p < 0.05). Using both scratch and transwell migration assays, we observed a positive correlation between CELSR1 expression and cell migratory capability. In addition, CELSR1 upregulation led to higher levels of tube formation in HAEC, while downregulating CELSR1 expression decreased tube formation (p < 0.05). Mechanistically, CELSR1-regulated migration and tube formation was mediated through disheveled segment polarity protein 3 (Dvl3). In conclusion, CELSR1 plays an important role in regulating multiple phenotypes of endothelial cells, including proliferation, migration, and formation of capillary-like structures.
Similar content being viewed by others
References
Formstone, C. J., Moxon, C., Murdoch, J., Little, P., and Mason, I. (2010) Basal enrichment within neuroepithelia suggests novel function(s) for CELSR1 protein, Mol. Cell. Neurosci., 44, 210–222.
Crompton, L. A., Du Roure, C., and Rodriguez, T. A. (2007) Early embryonic expression patterns of the mouse Flamingo and Prickle orthologues, Dev. Dyn., 236, 3137–3143.
Yates, L. L., Schnatwinkel, C., Murdoch, J. N., Bogani, D., Formstone, C. J., Townsend, S., Greenfield, A., Niswander, L. A., and Dean, C. H. (2010) The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis, Hum. Mol. Genet., 19, 2251–2267.
Cirone, P., Lin, S., Griesbach, H. L., Zhang, Y., Slusarski, D. C., and Crews, C. M. (2008) A role for planar cell polarity signaling in angiogenesis, Angiogenesis, 11, 347–360.
Descamps, B., Sewduth, R., Ferreira Tojais, N., Jaspard, B., Reynaud, A., Sohet, F., Lacolley, P., Allieres, C., Lamaziere, J. M., Moreau, C., Dufourcq, P., Couffinhal, T., and Duplaa, C. (2012) Frizzled 4 regulates arterial network organization through noncanonical Wnt/planar cell polarity signaling, Circ. Res., 110, 47–58.
Ju, R., Cirone, P., Lin, S., Griesbach, H., Slusarski, D. C., and Crews, C. M. (2010) Activation of the planar cell polarity formin DAAM1 leads to inhibition of endothelial cell proliferation, migration, and angiogenesis, Proc. Natl. Acad. Sci. USA, 107, 6906–6911.
Masckauchan, T. N., Agalliu, D., Vorontchikhina, M., Ahn, A., Parmalee, N. L., Li, C. M., Khoo, A., Tycko, B., Brown, A. M., and Kitajewski, J. (2006) Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2, Mol. Biol. Cell, 17, 5163–5172.
Yamada, Y., Fuku, N., Tanaka, M., Aoyagi, Y., Sawabe, M., Metoki, N., Yoshida, H., Satoh, K., Kato, K., Watanabe, S., Nozawa, Y., Hasegawa, A., and Kojima, T. (2009) Identification of CELSR1 as a susceptibility gene for ischemic stroke in Japanese individuals by a genomewide association study, Atherosclerosis, 207, 144–149.
Gouveia, L. O., Sobral, J., Vicente, A. M., Ferro, J. M., and Oliveira, S. A. (2011) Replication of the CELSR1 association with ischemic stroke in a Portuguese case-control cohort, Atherosclerosis, 217, 260–262.
Zhan, Y. H., Lin, Y., Tong, S. J., Ma, Q. L., Lu, C. X., Fang, L., Wei, W., Cai, B., and Wang, N. (2015) The CELSR1 polymorphisms rs6007897 and rs4044210 are associated with ischaemic stroke in Chinese Han population, Ann. Hum. Biol., 42, 26–30.
Curtin, J. A., Quint, E., Tsipouri, V., Arkell, R. M., Cattanach, B., Copp, A. J., Henderson, D. J., Spurr, N., Stanier, P., Fisher, E. M., Nolan, P. M., Steel, K. P., Brown, S. D., Gray, I. C., and Murdoch, J. N. (2003) Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse, Curr. Biol., 13, 1129–1133.
Devenport, D., Oristian, D., Heller, E., and Fuchs, E. (2011) Mitotic internalization of planar cell polarity proteins preserves tissue polarity, Nat. Cell Biol., 13, 893–902.
Tatin, F., Taddei, A., Weston, A., Fuchs, E., Devenport, D., Tissir, F., and Makinen, T. (2013) Planar cell polarity protein CELSR1 regulates endothelial adherens junctions and directed cell rearrangements during valve morphogenesis, Dev. Cell, 26, 31–44.
Maeder, M. L., Linder, S. J., Reyon, D., Angstman, J. F., Fu, Y., Sander, J. D., and Joung, J. K. (2013) Robust, synergistic regulation of human gene expression using TALE activators, Nat. Methods, 10, 243–245.
Livak, K. J., and Schmittgen, T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(–C(T)), Methods, 25, 402–408.
Van Meerloo, J., Kaspers, G. J., and Cloos, J. (2011) Cell sensitivity assays: the MTT assay, Methods Mol. Biol., 731, 237–245.
Justus, C. R., Leffler, N., Ruiz-Echevarria, M., and Yang, L. V. (2014) In vitro cell migration and invasion assays, J. Vis. Exp., 88, doi: 10.3791/51046.
Goodrich, L. V., and Strutt, D. (2011) Principles of planar polarity in animal development, Development, 138, 18771892.
Gray, R. S., Roszko, I., and Solnica-Krezel, L. (2011) Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity, Dev. Cell, 21, 120–133.
Seifert, J. R., and Mlodzik, M. (2007) Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility, Nat. Rev. Genet., 8, 126–138.
Chen, P. L., and Clandinin, T. R. (2008) The cadherin Flamingo mediates level-dependent interactions that guide photoreceptor target choice in Drosophila, Neuron, 58, 2633.
Usui, T., Shima, Y., Shimada, Y., Hirano, S., Burgess, R. W., Schwarz, T. L., Takeichi, M., and Uemura, T. (1999) Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled, Cell, 98, 585–595.
Joung, J. K., and Sander, J. D. (2013) TALENs: a widely applicable technology for targeted genome editing, Nat. Rev. Mol. Cell Biol., 14, 49–55.
Author information
Authors and Affiliations
Corresponding author
Additional information
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM16-021, April 3, 2016.
Rights and permissions
About this article
Cite this article
Zhan, YH., Luo, QC., Zhang, XR. et al. CELSR1 is a positive regulator of endothelial cell migration and angiogenesis. Biochemistry Moscow 81, 591–599 (2016). https://doi.org/10.1134/S0006297916060055
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297916060055