Abstract
Using Escherichia coli MG1655 lacIQ, ∆ackA-pta, ∆poxB, ∆ldhA, ∆adhE, ∆fadE, PL-SDφ10-atoB, Ptrc-ideal-4-SDφ10-fadB, PL-SDφ10-tesB, ∆yciA as a core strain, derivatives capable of synthesizing adipic acid from glucose through the inverted fatty acid β-oxidation pathway were obtained. Biosynthesis of the target compound by recombinants was ensured by the primary condensation of acetyl-CoA and succinyl-CoA by 3-oxoacyl-CoA thiolase PaaJ and the catalysis of the final reaction of the cycle by acyl-CoA dehydrogenases FadE and FabI. Deletion in the strains of sucCD genes encoding components of succinyl-CoA synthase did not increase the relative intracellular availability of succinyl-CoA for target biosynthetic reactions and did not lead to an increase in adipic acid accumulation by the recombinants. The secretion of succinic and malic acids by the strains with an impaired tricarboxylic acid cycle remained almost unchanged, indicating the activity in the cells of glyoxylate shunt reactions that compete with the cycle reactions for isocitrate, required for succinyl-CoA formation. When isocitrate lyase, malate synthases A and G, and bifunctional kinase/phosphatase of isocitrate dehydrogenase were inactivated in strains due to deletion of the aceBAK operon genes and glcB, adipic acid synthesis by recombinants increased three-fold and reached 0.33 mM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adipic acid is an industrially valuable chemical, which can serve as a convenient precursor in the subsequent synthesis of a wide range of high value-added substances, including lubricants, plasticizers, and pharmaceuticals. Meanwhile, most of the adipic acid produced annually in amounts exceeding 3.5 million tons is used to obtain nylon-6,6 [1]. At present, adipic acid production is based on petrochemical synthesis with benzene used as a precursor [2]. The method implying the primary biotechnological production of cis,cis-muconic acid from renewable carbon sources, followed by its catalytic conversion into adipic acid has been suggested as ecologically reasonable alternative [3]. Indeed, the feasibility of microbiological conversion of glucose and glycerol into cis,cis-muconic acid has been demonstrated using directly engineered strains of microorganisms traditionally used in industrial biotechnology, such as Eschericha coli and Saccharomyces cerevisiae [4–7]. In addition, the potential pathway of direct biocatalytic conversion of substrate into adipic acid through the intermediate formation of 2-oxoadipate, which involves the primary condensation of acetyl-CoA with 2-oxoglyutarate, has been proposed [8]. However, experimental implementation of the respective concept has not so far been demonstrated. A promising alternative to α-reduction of 2-oxoadipate is adipic acid production from adipyl-CoA formed resulting from the reversal of the biochemical reactions of β-oxidation including, inter alia, the reversed reactions of phenylacetate or fatty acid degradation. The formation of an appropriate CoA precursor in the sequence of such reactions involves the primary condensation of acetyl-CoA and succinyl-CoA, followed by the conversion of 3-oxoadipyl-CoA to 3-hydroxyadipyl-CoA, then to 2,3-didehydroadipyl-CoA and, finally, to adipyl-CoA. Thioester bond hydrolysis resulting in the formation of target compound can be catalyzed in this case by thioesterases with the appropriate substrate specificity. The synthesis of adipic acid from glucose in E. coli as a result of the reversal of phenylacetate degradation pathway has recently been successfully demonstrated upon the overexpression in the core strains of both the native genes of the paa-operon and auxiliary genes of other organisms [8–11]. The thioesterase genes from Acinetobacter baylyi [8] and Mus musculus [10, 11], as well as the acyl-CoA dehydrogenase/enoyl-CoA reductase genes from Clostridium acetobutylicum [10] and Treponema denticola [9, 11] were primarily used as such auxiliary genes. At the same time, adipic acid biosynthesis by E. coli upon the catalysis of the reactions of adipyl-CoA formation by the enzymes of fatty acid β-oxidation (FABO) has not been shown.
Since the efficiency of subsequent reactions of reverse β-oxidation depends directly on the efficiency of the primary stage of 3-oxoadipyl-CoA formation, the attempts were made to increase the intracellular availability of succinyl-CoA in the respective adipate-secreting recombinants. This metabolite is an intermediate of the tricarboxylic acid cycle and, in this regard, the approaches to increasing its intracellular availability for the reactions of reverse β-oxidation were based, first of all, on the prevention of utilization of succinyl-CoA in the subsequent reactions of the cycle due to inactivation of succinate dehydrogenase (EC 1.3.5.1) [9] or succinyl-CoA synthetase (EC 6.2.1.5) [12]. At the same time, it is known that the activity of 2-oxoglutarate dehydrogenase (EC 1.2.4.2) responsible for succinyl-CoA formation in E. coli cells is decreased during the growth in glucose-containing media [13], and one of the key precursors of the respective thioester, isocitrate, can be involved in the glyoxylate shunt activated during intensive formation of acetyl-CoA in a cell [14].
This work was aimed at constructing E. coli strains capable of adipic acid biosynthesis from glucose with the involvement of the enzymes of fatty acid β-oxidation, and assessing the effects of glyoxylate shunt inactivation on the biosynthesis of a target compound by recombinants.
MATERIALS AND METHODS
Reagents. The restriction endonuclease BglII, Taq DNA polymerase, T4 DNA ligase (Thermo Scientific, Lithuania), and Kapa HiFi DNA polymerase (Roche, Switzerland) were used in the work. PCR products were purified by agarose gel electrophoresis and isolated with a QIAquick Gel Extraction Kit (Qiagen, United States). Oligonucleotides (Evrogen, Russia) are presented in Table 1. Nutrient medium components, salts and other reagents were produced by Panreac (Spain) and Sigma (United States).
Bacterial strains, plasmids and media. The strain E. coli K-12 MG1655 (VKPM B-6195) and the previously constructed strains E. coli BOX3.1 Δ4 Ptrc-id-4-fadE and BOX3.1 Δ4 Ptrc-id-4-fabI [15] with modified regulation of expression of the genes encoding the key enzymes of aerobic fatty acid β-oxidation and thioesterase II, as well as lacking the pathways of mixed acid fermentation and the activity of nonspecific thioesterase YciA, were used as the core ones to construct all strains obtained in the work. The bacterial strains and plasmids used in the work are presented in Table 2. The bacteria were cultivated in LB, SOB, and SOC complete media and M9 minimal medium [16], with the addition of ampicillin (100 µg/mL) or chloramphenicol (30 µg/mL) if necessary.
Strain construction. Target modifications were introduced into the E. coli chromosome using the technique described previously [17].
The DNA fragment for replacing the native regulatory region of the paaJ gene by artificial genetic element PL-SDφ10 containing the PL lambda phage promoter and the efficient ribosome binding site of the T7 phage gene φ10 was constructed in several stages. At the first stage, PCR was used to obtain a DNA fragment containing the BglII recognition site, the PL promoter, the SD sequence of the T7 phage gene φ10, and 36 nucleotides complementary to the 5'-end of the coding region of the paaJ gene.
The fragment was obtained in two stages. At the first stage, a DNA fragment containing the BglII recognition site, the PL promoter, and part of the SD sequence of the T7 phage gene φ10 was obtained by PCR using the primers P1 and P2 and the genomic DNA of phage lambda as a template. The resulting PCR product served as a template in the next round of PCR using the P1 and P3 primers. The P3 primer contained a region complementary to the 3'-end of the PL promoter, the SD sequence of the T7 phage gene φ10, and the first 36 nucleotides from the reading frame of the paaJ gene. The second stage of DNA fragment construction was performed in parallel. The DNA fragment containing the BglII recognition site, the chloramphenicol resistance marker (the cat gene) and 36 nucleotides homologous to the DNA region upstream the coding region of the paaJ gene was obtained by PCR with primers P4 and P5 and the pMW118-(λattL-Cm-λattR) plasmid [18] used as a template. The resulting DNA fragments were treated with the BglII restriction endonuclease and ligated by T4 DNA ligase. The ligation product was amplified using primers P3 and P5. The resulting PCR product was integrated into the chromosome of E. coli strain MG1655 carrying the pKD46 helper plasmid [17]. The matching of the planned and experimentally obtained nucleotide sequences of the new regulatory element inserted upstream of the coding region of the paaJ gene was confirmed by the sequencing with P6 and P7 primers.
The linear DNA fragment for inactivation of the sucCD genes, containing the chloramphenicol resistance marker (the cat gene), was obtained by PCR with the P8 and P9 primers and the pMW118-(λattL-Cm-λattR) plasmid used as a template. The resulting DNA fragment was integrated into the chromosome of the E. coli strain MG1655 carrying the pKD46 helper plasmid. The inactivation of the sucCD genes in the chromosomes of selected integrants was confirmed by PCR analysis using the locus-specific primers P10 and P11.
The corresponding individual genetic modifications were inserted into the chromosomes of the target recombinant strains by P1-dependent transductions [16]. The previously obtained preparations of P1-transducing phages containing the respective marked modifications were used in case of inactivation of the aceBAK and glcB genes [19]. Removal of the marker flanked by the att-sites of phage lambda from the chromosomes of target strains was performed using the pMWts-Int/Xis plasmid as described [20]. The transformation of strains with plasmids was carried out according to the standard method.
Cultivation of the strains. The recombinant strains were grown overnight in M9 medium containing 2 g/L glucose at 37°C. For microaerobic cultivation, 5 mL of the resulting overnight cultures were diluted 10 times by the addition of 45 mL of M9 medium containing 10 g/L glucose, 10 g/L yeast extract and 2.5 g/L NaHCO3. The resulting cultures were incubated in 750-mL flasks closed with cotton plugs on a rotary shaker at 250 rpm for 8 h at 37°C. Oxygen saturation of the medium was assessed in the control flasks with the respective cultures incubated in the presence of resazurin. To induce the expression of genes under the control of the LacI-dependent promoter Ptrc-ideal-4, isopropyl-β-D-thiogalactoside (IPTG) was added to the culture media 3 h after the start of incubation to a final concentration of 1.0 mM.
Cell suspensions were centrifuged at 10 000 g for 10 min and the concentrations of secreted metabolites and residual glucose were determined in the supernatants. All experiments were performed in no less than three replicates.
Analytical methods. The concentrations of organic acids in culture liquids freed from biomass by centrifugation were determined by HPLC using a Waters HPLC system (United States). A Rezex ROA-Organic Acid H+ (8%) ion-exclusion column (Phenomenex, United States) with detection at a wavelength of 210 nm was used. An aqueous solution of sulfuric acid (2.5 mM) with a flow rate of 0.5 mL/min was used as the mobile phase. For glucose concentration measurements, the system was equipped with a Waters 2414 refractive index detector and a Spherisorb-NH2 column (Waters, United States). An acetonitrile–water mixture (75/25 vol/vol) at a flow rate of 1.0 mL/min was used as the mobile phase.
The identification and quantification of butyric and adipic acids in culture liquids were performed by the method of gas chromatography–mass spectrometry. Sample preparation included the extraction of target analytes from culture liquid, evaporation of the extract, and derivatization with the formation of trimethylsilyl derivatives. Liquid–liquid microextraction with ethyl acetate was used to extract the target compounds. Prior to the extraction, a sample aliquot was mixed with the aqueous solutions of internal standards (valeric and pimelic acids) and 2 M sodium chloride solution and adjusted to pH 1–2 using 2 M hydrochloric acid. Ethyl acetate extract was mixed with dry sodium sulfate and centrifuged; the resulting supernatant was evaporated in a vacuum evaporator to the minimum volume at 30°C. Silylation was performed by incubating the residue after the stage of evaporation with N,O-bis(trimethylsilyl)trifluoroacetamide containing 1% trimethylchlorosilane at 60°С for 15 min.
Chromatography–mass spectrometry was performed using an Agilent 6890N gas chromatograph equipped with a 7683B autosampler and an Agilent 5975 MSD mass-selective detector (Agilent, United States). The system was equipped with an Agilent DB-5MS capillary column of 30 m in length, with an inner diameter of 0.25 mm and a film thickness of 0.25 µm. Helium served as a carrier gas at a constant flow rate of 1.0 mL/min. A 1-µL sample was injected into the evaporator in the split injection mode at a split ratio of 1 : 10. The evaporator temperature was 230°C. The temperature program of the column oven was as follows: the initial isotherm of 2 min at 60°C, followed by the linear gradient to 200°C at a rate of 5°C/min, then to 250°C at a rate of 15°C/min, and the final isotherm of 5 min at 250°C. Analytes were ionized by electron ionization (70 eV). The temperature of the ion source of the mass spectrometer was 230°C. The temperature of the mass spectrometer interface was 250°C. The mass selective detector was operated in the SIM mode (145 m/z and 73 m/z for butyric acid, 275 m/z and 172 m/z for adipic acid, 159 m/z for valeric acid, and 289 m/z for pimelic acid). The data were collected and processed using the Agilent MSD ChemStation software. The analytical system was calibrated within a range of 0.01–0.10 mg/mL for both analytes with a detection limit of 0.002 mg/mL. Weighted linear regression was used for the analysis of the calibration data, and the coefficient of determination R2 = 0.993 was obtained. The target analytes were identified by comparing the data obtained with the characteristics of the respective standards (retention time and mass spectrum).
RESULTS AND DISCUSSION
The previously engineered E. coli strains BOX3.3 Δ4 Ptrc-id-4-fadE and BOX3.3 Δ4 Ptrc-id-4-fabI were used as the core strains for the construction of derivatives capable of adipic acid biosynthesis from glucose with the participation of FABO enzymes and for the further assessment of the effect of glyoxylate shunt inactivation on the biosynthesis of the target compound by recombinants (Table 2) [15]. In these strains, the mixed acid fermentation pathways competing with the reactions of reversed FABO for the key precursor metabolites, pyruvic acid and acetyl-CoA, were inactivated due to deletions of the ackA, pta, poxB, ldhA and adhE genes. The activity of enzymes capable of catalyzing the reactions of reversed FABO was increased in the strains due to replacement of native regulatory regions of the respective genes, atoB, fadB, fadE and fabI, by efficient artificial regulatory elements Ptrc-ideal-4-SDφ10 and PL-SDφ10. In addition, to ensure the convertion of the terminal CoA products of the reversed FABO into the respective carboxylic acids, the expression of the thioesterase II gene tesB was increased in the strains, while the gene encoding nonspecific thioesterase YciA was deleted in order to prevent unwanted hydrolysis of the thioester bond of the intermediates of reversed cycle. As a result, during test-tube fermentation, the strains were capable of synthesizing up to ~0.5 mM of butyric acid from glucose resulting from a one-turn functional reversal of FABO [15] and could serve as an appropriate chassis for the construction of model adipic acid producers.
Stoichiometrically balanced biosynthesis of two adipic acid molecules from three glucose molecules through the reversed FABO implies the consumption of four NADH molecules. At the same time, glycolytic generation of necessary precursors ensures the formation of six respective reducing equivalents; aerobic conversion of four pyruvic acid molecules into acetyl-CoA provides another four equivalents; the subsequent formation of two succinyl-CoA in the TCA cycle reactions is also accompanied by the formation of four additional NAD(P)H. However, during aeration, most of the reducing equivalents formed via substrate catabolism are oxidized by the respiratory electron transport chain with oxygen as the terminal electron acceptor. On the other hand, during anaerobiosis, despite the decreased formation of reducing equivalents due to the conversion of pyruvic acid into acetyl-CoA by pyruvate formate lyase (EC 2.3.1.54), the intracellular redox status will prevent not only the biosynthesis of the target compound but also efficient substrate consumption resulting from the inhibition of glycolysis by an excessive intracellular NADH pool [21]. Thus, neither fully anaerobic nor fully aerobic conditions could be considered optimal for adipic acid biosynthesis from glucose via the reversed FABO by the previously constructed BOX3.3 Δ4 Ptrc-id-4-fadE and BOX3.3 Δ4 Ptrc-id-4-fabI strains. Hence, the biosynthetic potential of the strains in the present work was assessed during microaerobic cultivation. The media were also supplemented with 2.5 g/L NaHCO3 to increase the intensity of formation of oxaloacetic acid (OAA), which is necessary for the involvement of acetyl-CoA in the TCA cycle reactions leading to the formation of succinyl-CoA.
Under the respective cultivation conditions, the core strains did not secrete detectable amounts of adipic acid, despite the presence of a CO2 source in the medium (Table 3). This could be due both to the insufficient activity of the TCA cycle generating succinyl-CoA and to the substrate specificity of 3-oxoacyl-CoA-thiolase AtoB (EC 2.3.1.16) responsible for the primary condensation of acetyl- and acyl-CoA in the strains. At the same time, the presence of significant amounts of succinic acid in the culture media (Table 3) were rather in favor of the latter assumption. Indeed, in the previous works [8–11], adipic acid biosynthesis through reversed β-oxidation by directly engineered E. coli strains was achieved upon the overexpression in recombinants of the gene of another native thiolase, PaaJ (EC 2.3.1.174), with the confirmed ability to form 3-oxoadipyl-CoA. In this regard, the paaJ gene expression was increased in the core strains BOX3.3 Δ4 Ptrc-id-4-fadE and BOX3.3 Δ4 Ptrc-id-4-fabI resulting from the replacement of its native regulatory region with artificial genetic element PL-SDφ10 carrying a strong constitutive promoter of phage lambda and an efficient ribosome binding site of T7 phage gene 10.
The respective BOX3.3 Δ4 Ptrc-id-4-fadE PL-paaJ and BOX3.3 Δ4 Ptrc-id-4-fabI PL-paaJ strains during microaerobic utilization of glucose secreted about 100 µM of adipic acid to the medium, with a proportional decrease in the accumulation of butyric acid (Table 3). At the same time, as in the case of butyric acid, the formation of the six-carbon adipic acid by the BOX3.3 Δ4 Ptrc-id-4-fabI strain was slightly lower compared to the BOX3.3 Δ4 Ptrc-id-4-fadE strain. This observation ran counter to the previous data on preferential formation, as a result of functional reversal of FABO, of six- and eight-carbon carboxylic acids by the strains expressing FabI as an acyl-CoA dehydrogenase [15]. Nevertheless, the previous results were obtained in the case of production by recombinants of monocarboxylic acids and may not reflect the specificity of particular enzymes to the respective CoA derivatives containing the ω-carboxylic group.
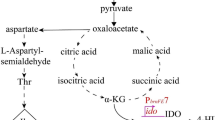
It should be noted that succinic acid production by the strains with enhanced expression of paaJ did not decrease, indicating either inability of the strains to efficiently involve the corresponding ω-functionalized thioester, succinyl-CoA, in the reversed FABO reactions or its decreased availability in recombinants. Succinyl-CoA is formed in the TCA cycle from 2-oxoglutarate by 2-oxoglutarate dehydrogenase and further channeled to subsequent reactions of the cycle by succinyl-CoA synthetase. Thus, inactivation of the latter enzyme could potentially increase the intracellular availability of succinyl-CoA for the target reactions of reversed FABO.
Inactivation of the sucCD genes encoding the components of succinyl-CoA synthetase in the strains BOX3.3 Δ4 Ptrc-id-4-fadE PL-paaJ and BOX3.3 Δ4 Ptrc-id-4-fabI PL-paaJ did not change the production of the target compound by the recombinants, while the levels of succinic acid accumulation by the strains slightly decreased (Table 3). However, this decrease was only about 20%, indicating an insignificant contribution of succinyl-CoA synthetase activity to the formation of this dicarboxylate. It is known that the expression of the genes of the sucABCD operon, which encode the components of 2-oxoglutarate dehydrogenase and succinyl-CoA synthetase, is drastically decreased in E. coli cells growing in rich and glucose-containing media [13]. Thus, glyoxylate shunt reactions appeared to be responsible for succinic acid production by the BOX3.3 Δ4 Ptrc-id-4-fadE PL-paaJ and BOX3.3 Δ4 Ptrc-id-4-fabI PL-paaJ strains. The secretion of notable amounts of malic acid by the recombinants confirmed this assumption. The key enzymes that catalyze the reactions of the glyoxylate shunt, isocitrate lyase (EC 4.1.3.1) and malate synthase A (EC 2.3.3.9), are encoded in E. coli cells by the aceA and aceB genes comprising the aceBAK operon together with the aceK gene encoding the bifunctional isocitrate dehydrogenase kinase/phosphatase. The latter enzyme regulates the activity of isocitrate dehydrogenase, redistributing the carbon flux between the oxidative TCA cycle and the glyoxylate shunt [22]. The expression of genes of the aceBAK operon undergoes complex transcriptional regulation, including action of proteins such as IclR, FadR, FruR, ArcAB, and CRP-cAMP [23, 24]. Under glucose abundant conditions, the expression of the aceBAK operon genes is repressed by a specific transcriptional regulator IclR. At the same time, upon decreased glucose availability, the expression of the operon is activated by the global regulator CRP-cAMP [24]. Moreover, the increased acetyl-CoA formation in cells also contributes to the activation of transcription of the aceBAK operon [23]. Hence, it should be noted that the studied strains synthesized considerable amounts of acetic acid (Table 3), suggesting intracellular accumulation of elevated levels of acetyl-CoA which, in the absence of phosphotransacetylase Pta, acetate kinase AckA and nonspecific thioesterase YciA in the cells, was converted into the respective derivative by other cellular enzymes possessing thioesterase activity such as the YciA, YbgC and YdiI proteins with a broad substrate specificity toward acyl derivatives of coenzyme A [25–27]. Thus, given that the glyoxylate shunt reactions leading to the production of succinic and malic acids proceed without the intermediate formation of succinyl-CoA, yet consuming its precursor, isocitrate, the genes of the aceBAK operon were deleted in the BOX3.3 Δ4 Ptrc-id-4-fadE PL-paaJ and BOX3.3 Δ4 Ptrc-id-4-fabI PL-paaJ strains. The glcB gene encoding malate synthase G was also deleted in the strains.
As a result of introduction of respective genetic modifications into the strains, the secretion of malic acid by the BOX3.3 Δ4 Ptrc-id-4-fadE PL-paaJ ΔaceBAK ΔglcB and BOX3.3 Δ4 Ptrc-id-4-fabI PL-paaJ ΔaceBAK ΔglcB ceased, while the accumulation of succinic acid decreased four times (Table 3). Concomitantly, the strains synthesized adipic acid in the amounts that increased by three times, up to ~0.3 mM, along with a dramatic decrease in the formation of butyric acid by the recombinants. Thus, inactivation of the glyoxylate shunt promoted biosynthesis of the target compound by the recombinants by favoring succinyl-CoA formation in the reactions of oxidative TCA cycle excluding the involvement of the precursor in reactions unrelated to the formation of the corresponding thioester. Indeed, persistent secretion of succinic acid by the strains devoid of the glyoxylate shunt activity indicated the production of this dicarboxylate in the TCA cycle from succinyl-CoA formed as a result of action of 2-oxoglutarate dehydrogenase. At the same time, the observed residual formation of butyric acid by the constructed strains suggested incomplete involvement of succinyl-CoA in the reactions of reversed FABO. This could be accounted for both by the nonoptimal substrate specificity of the enzymes involved in formation of the target compound and by insufficient levels of their activity in cells. In accordance with the rational metabolic engineering approaches, such problems can be solved both by fine tuning of targeted gene expression and by selection of alternative target genes for their expression in the promising recombinants.
The current study resulted in construction of E. coli strains capable of adipic acid biosynthesis from glucose through the reversed FABO pathway. The positive effect of inactivation of the glyoxylate shunt on biosynthesis of the target compound by the recombinants has been shown. The significance of intracellular availability of succinyl-CoA formed in the TCA cycle reactions for efficient biosynthesis of the final product by the reversed FABO has been demonstrated. The achieved levels of adipic acid synthesis exceeded the respective levels of the synthesis of butyric acid simultaneously formed by the strains through reversed fatty acid β-oxidation without the involvement of succinyl-CoA.
Change history
12 August 2023
An Erratum to this paper has been published: https://doi.org/10.1134/S0003683823320029
REFERENCES
Lang, M. and Li, H., ChemSusChem, 2022, vol. 15, no. 1, p. e202101531. https://doi.org/10.1002/cssc.202101531
Skoog, E., Shin, J.H., Saez-Jimenez, V., Mapelli, V., and Olsson, L., Biotechnol. Adv., 2018, vol. 36, no. 8, pp. 2248–2263.
Thomas, J.M., Raja, R., Johnson, B.F., O’Connell, T.J., Sankar, G., and Khimyak, T., Chem. Commun., 2003, vol. 21, no. 10, pp. 1126–1127.
Lin, Y., Sun, X., Yuan, Q., and Yan, Y., Metab. Eng., 2014, vol. 23, pp. 62–69.
Zhang, H., Li, Z., Pereira, B., and Stephanopoulos, G., Microb. Cell. Factories, 2015, vol. 14, no. 1. https://doi.org/10.1186/s12934-015-0319-0
Weber, C., Brueckner, C., Weinreb, S., Lehr, C., Essl, C., and Boles, E., Appl. Environ. Microbiol., 2012, vol. 78, pp. 8421–8430.
Curran, K.A., Leavitt, J.M., Karim, A.S., and Alper, H.S., Metab. Eng., 2013, vol. 15, pp. 55–66.
Kallscheuer, T., Gatgens, J., Lubcke, M., Pietruszka, J., Bott, M., and Polen, T., Appl. Microbiol. Biotechnol., 2017, vol. 101, no. 6, pp. 2371–2382.
Yu, J.L., Xia, X.X., Zhong, J.J., and Qian, Z.G., Biotechnol. Bioeng., 2014, vol. 111, no. 12, pp. 2580–2586.
Babu, T., Yun, E.J., Kim, S., Kim, D.H., Liu, K.H., Kim, S.R., and Kim, K.H., Proc. Biochem., 2015, vol. 50, no. 12, pp. 2066–2071.
Cheong, S., Clomburg, J.M., and Gonzalez, R., Nat. Biotechnol., 2016, vol. 34, no. 5, pp. 556–561.
Zhao, M., Huang, D., Zhang, X., Koffas, M.A.G., Zhou, J., and Deng, Y., Metab. Eng., 2018, vol. 47, pp. 254–262.
Park, S.J., Chao, G., and Gunsalus, R.P., J. Bacteriol., 1997, vol. 179, pp. 4138–4142.
Skorokhodova, A.Y., Gulevich, A.Y., Morzhakova, A.A., Shakulov, R.S., and Debabov, V.G., Biotechnol. Lett., 2013, vol. 35, no. 4, pp. 577–583.
Gulevich, A.Yu., Skorokhodova, A.Yu., and Debabov, V.G., Appl. Biochem. Microbiol., 2022, vol. 58, no. 4, pp. 361–367.
Sambrook, J., Fritsch, E., and Maniatis, T., Molecular Cloning: A Laboratory Manual, New York: Cold Spring Harbor Lab. Press, 1989, 2nd ed.
Datsenko, K.A. and Wanner, B.L., Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, no. 12, pp. 6640–6645.
Katashkina, Zh.I., Skorokhodova, A.Yu., Zimenkov, D.V., Gulevich, A.Yu., Minaeva, N.I., Doroshenko, V.G., Biryukova, I.V., and Mashko, S.V., Mol. Biol. (Moscow), 2005, vol. 39, no. 5, pp. 719–726.
Skorokhodova, A.Yu., Gulevich, A.Yu., and Debabov, V.G., Appl. Biochem. Microbiol., 2018, vol. 54, no. 3, pp. 245–251.
Gulevich, A.Yu., Skorokhodova, A.Yu., Ermishev, V.Yu., Krylov, A.A., Minaeva, N.I., Polonskaya, Z.M., Zimenkov, D.V., Biryukova, I.V., and Mashko, S.V., Mol. Biol. (Moscow), 2009, vol. 43, no. 3, pp. 505–514.
Vemuri, G.N., Altman, E., Sangurdekar, D.P., Khodursky, A.B., and Eiteman, M.A., Appl. Environ. Microbiol., 2006, vol. 72, pp. 3653–3661.
Laporte, D.C., Stueland, C.S., and Ikeda, T.P., Biochimie, 1989, vol. 71, nos. 9–10, pp. 1051–1057.
Cronan, J.E. and Laporte, D., Tricarboxylic acid cycle and glyoxylate bypass, in Escherichia coli and Salmonella: Cellular and Molecular Biology, Neidhardt, F., Ed., Washington: ASM Press, 1996, pp. 206–216.
Waegeman, H., Beauprez, J., Moens, H., Maertens, J., De Mey, M., Foulquie-Moreno, M.R., Heijnen, J.J., Charlier, D., and Soetaert, W., BMC Microbiol., 2011, vol. 11, no. 70. https://doi.org/10.1186/1471-2180-11-70
Clomburg, J.M., Vick, J.E., Blankschien, M.D., Rodriguez-Moya, M., and Gonzalez, R., ACS Synth. Biol., 2012, vol. 1, pp. 541–554.
Zhuang, Z., Song, F., Zhao, H., Li, L., Cao, J., Eisenstein, E., Herzberg, O., and Dunaway-Mariano, D., Biochemistry, 2008, vol. 47, no. 9, pp. 2789–2796.
Chen, M., Ma, X., Chen, X., Jiang, M., Song, H., and Guo, Z., J. Bacteriol., 2013, vol. 195, no. 12, pp. 2768–2775.
Funding
The work was supported by the Russian Science Foundation (project no. 22-14-00040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Makeeva
The original online version of this article was revised: Due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gulevich, A.Y., Skorokhodova, A.Y. & Debabov, V.G. The Effect of Glyoxylate Shunt Inactivation on Biosynthesis of Adipic Acid through Inverted Fatty Acid β-Oxidation by Escherichia coli Strains. Appl Biochem Microbiol 59, 267–274 (2023). https://doi.org/10.1134/S0003683823030080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683823030080