Abstract
Around the world, there are persistent and growing health inequalities both between and within countries. The U.K. Government’s flagship policy for addressing inequalities is called ‘Levelling Up’. One of its missions is to narrow the gap in healthy life expectancy (HLE) between the healthiest and unhealthiest areas in England and to improve overall HLE by 5 years by 2035. We show that smoking is one of the major causes of health inequalities. We find a 17-year difference in HLE between local authorities, and that the number of years spent in ill health tended to be greatest in areas with the highest mortality from smoking-related disease. Our aim is to see if the 5-year target could be achieved, assuming there were drastic controls on the sale and consumption of tobacco. We show that never smokers enjoy six more years of good health at age 20 than current or ex-smokers. A complete ban on smoking would lead to a 2.5-year improvement in HLE, and also lengthen the working lives of both men and women. We conclude that while a complete tobacco ban is significant, other public health measures are needed for the full achievement of the target. The paper briefly considers wider issues and suggestions for further research and its international significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Around the world, there are persistent and growing health inequalities, both between and within countries, to the detriment of economies and society as a whole. The U.K. is a world leader in health inequalities research, yet this research constantly demonstrates how pernicious health inequalities in the U.K. are and how difficult it is to make a difference. There is a need to share experience and learn lessons about what measures are available and the policies being adopted to assess their implementation and effectiveness in different international settings.
The U.K. Government published a seminal white paper on Levelling Up in 2022 (Department for Levelling Up Housing and Communities 2022), where 12 wide-ranging missions for levelling up the U.K. were laid out. The only health-related mission involved narrowing the gap in Healthy Life Expectancy (HLE) between local areas by 2030 and increasing HLE by 5 years by 2035 (Department for Levelling Up Housing and Communities 2022). Within this mission, there was also a strong focus on prevention and on health disparities by ethnicity, socioeconomic background and geographical region.
It proposed that communities with higher prevalence of health behavioural risk factors like smoking or poor diet, and where access to health services is more limited, would receive special attention from the government to reduce health inequalities. Inequality is defined by the United Nations as ‘the state of not being equal, especially in status, rights, and opportunities’, although it is acknowledged to have different meanings for different people (Development Strategy and Policy Analysis Division United Nations 2015). More specifically, the U.K. National Health Service (NHS) defines health inequality as ‘unfair and avoidable differences in health across the population, and between different groups within society’ (NHS England 2023), which is clearly captured by differences in HLE between groups of people and geographical areas.
From a policy perspective, the evidence base and decisions on resource allocation are of greatest concern. Similar to the myriad interpretations of inequality, policymakers can appeal to different, contrasting theories of distributive justice in political philosophy (Binns 2018). These theories largely support the redistribution of resources; however, unlike other resource-related theories, levelling up presupposes a net input of resources to improve people’s lives, rather than a redistribution of existing resources, and is therefore potentially more resource-intensive or requires greater political will. Levelling up policies specifically appeal to Rawls’ theory of ‘making the worst off as well off as possible’, which does not necessarily imply a detrimental impact on those who are originally better off.
This ambition to increase HLE in the U.K. is not new. In fact, its origins go as far back as the U.K. Conservative Party Manifesto in 2019 (Public Services Committee 2020). Subsequently, the All-Party Parliamentary Group (APPG) for Longevity picked up the specific target of ‘extending HLE by 5y by 2035’ and amplified it around 2020. The APPG later added the second target to ‘narrow the gap in HLE by 2035’ in 2020–2021 (Green et al. 2021). The Levelling Up white paper then moved the date of this second target closer, from 2035 to 2030 (Department for Levelling Up Housing and Communities 2022).
Whether these growing ambitions, particularly in reducing health inequalities and not merely improving health, were matched by policies on the ground remains to be seen. The government has pledged to tackle the core drivers of inequalities in health outcomes mentioned in this white paper, visibly through reorganising its agencies to create the Office for Health Improvement and Disparities (OHID) and the Department for Levelling Up, Housing and Communities, and through the white paper on health disparities that was due in 2022 (Department for Levelling Up Housing and Communities 2022) but has been delayed.
From a wider perspective, health inequalities are also in part driven by geographical differences in population age structures, alcohol consumption and diet, and are affected by wider societal factors e.g. cultural norms, quality of housing and access to healthcare. Within the U.K., tobacco use, unhealthy diet, alcohol consumption and physical inactivity are among the key drivers where there is scope for intervention, as they are socioeconomically patterned (Marteau et al 2019). Following previous interventions, there has been a secular decline in tobacco use, particularly over the last few decades in the U.K. (Everest et al. 2022), which coincides with increasing LE and HLE over the same period, indicating a large measure of success.
A widening gap between LE and HLE means people survive for longer but also spend more of their lives on average in poor health, and so anything that reduces that gap will be beneficial to society. Since 2000, LE has increased by more years than HLE, and therefore in the ill health gap has been increasing (Department for Levelling Up Housing and Communities 2022). Is this simply due to the success of the healthcare system in keeping people alive for longer or a widening in inequalities? Added to this, previous advances in LE over many decades have stalled, which contributes to the view that something fundamental has changed.
The key point is that preventing people from becoming ill in the first place is more effective than treating the causes. We know, for example, that areas where people have the best health are economically more productive, as well as better off financially. It follows, therefore, that health improvement leads to greater economic productivity, but reducing health inequalities between smaller geographical areas is a particular challenge. We know that some risk factors are more closely related than others e.g. if they are aetiologically, socially or geographically proximal. Recent research by the International Longevity Centre (ILC) found that the economic cost of smoking is about GBP 19.1 billion a year in reduced economic output due to shortened working lives—though this excludes wider socioeconomic harms to families and communities and savings in healthcare costs and welfare benefits (Mayhew and Dimitriadis 2021).
In 2019, the U.K. Government set the ambition for England to be ‘Smoke free by 2030’, and this was followed in June 2022 by the Khan review ’Making smoking obsolete’ (Office for Health Improvement and Disparities 2022), which laid out recommendations for smoking interventions and smoking cessation policies. Smoking is often cited as reducing LE by up to 10 years (Doll et al. 2004). Although nationwide smoking prevalence has fallen to 14%, from 80% among men in the 1950s, it remains the largest cause of preventable mortality (Everest et al. 2022), and affects ex-smokers as well as smokers. Smoking is a primary cause of death from cancer, heart and respiratory disease, accounting for about 78,000 deaths a year (Mayhew and Dimitriadis 2021) and 0.5 million smoking-related hospital admissions (Office for Health Improvement and Disparities 2023b).
Of key importance and utmost relevance to policy, what difference could smoking cessation make towards the government’s target of improving HLE by 5 years by 2035? In this paper, we investigate what gains in HLE could be achieved in this time horizon, what the health and mortality impacts would be and how long they would take to materialise. We touch on some of the associated questions, such as how the health benefits would be distributed, for example, between geographical areas, men and women and age groups. Given the ambitious Levelling Up mission for HLE, we investigate these impacts for the scenario where there are drastic controls on the sale and consumption of tobacco.
How smoking habits have changed
Smoking tobacco has been practised for centuries, but the sale of cigarettes only really took off during the First World War after the process of mass-producing cigarettes was mechanised in the late 1880s. The first research demonstrating that smoking causes lung cancer deaths was published in the 1950s (Doll and Hill, Smoking and carcinoma of the lung; preliminary report 1950). Figure 1 illustrates how smoking prevalence has changed from the 1970s onwards and the timing of larger-scale and policy interventions aimed at reducing smoking prevalence since the scientific acceptance that a causal link exists.
Smoking cessation policies and trends in smoking prevalence over 1970s–2020. The most recent 10-year duration to 2020 has been marked out to extrapolate a 2020–2030 trajectory, in the context of the U.K. Government ‘Smoke free by 2030’ goal (adapted from Everest et al. 2022)
Generally, the chart suggests that it is possible to reduce consumption by means of various interventions such as price controls, banning tobacco advertising and banning smoking in public places, if some degree of causality via time trends is assumed. Each key policy or intervention coincides with a reduction in smoking prevalence a few years later, although the pace of reduction has varied. For example, higher tobacco duties introduced between 1993 and 2000 do not appear to have had as much effect as introducing health warnings on packaging in 1986 or bans on smoking in public places.
Figure 1 does not reflect that e-cigarettes were introduced in the early 2000s and are now widely used. This has therefore potentially affected this trend and is an issue to which we return later. In 2022, which is subsequent to this chart, the government review (‘Smoke free by 2030’) recommends a multi-pronged set of interventions, which includes a set of 15 public health, fiscal and clinical initiatives. None are particularly new except the proposal of raising the age of tobacco sale and offering vaping as a substitute to smoking. Further discussion on the efficacy of policies either under consideration or implemented is also covered in a later section, in order to assess the lessons learned so far and what might be feasible in the near future.
What this brief overview shows is that there has been progress in cutting smoking prevalence but that eliminating it altogether is likely to be a long, drawn-out process. The last major policy change in 2015 was banning smoking of cigarettes in cars in the presence of children, but what further steps can be taken is unclear without more restrictions on the use or purchase of tobacco. Our aim is to understand how smoking affects the general population at all ages and what difference a complete cessation of smoking would make.
In the next section, we set out our approach, which considers the difference smoking makes not only to LE but also to health. In the third section, we analyse how differences in smoking prevalence between different parts of the U.K. are linked to health inequalities; in the fourth section, we analyse the association between smoking and other risk factors that may reinforce unhealthy behaviours and so increase the risk of death. The fifth section briefly compares alternative approaches to assessing the impact of smoking cessation, which includes other risk factors such differences in education levels, while the penultimate section examines what can be learned from research and policies in other countries. The final section concludes.
Measures of life, health and work span
In what follows, we build on three fundamental measurements: LE, state-of-health expectancy and working life expectancy as our key indicators of well-being and work capability. LE is defined as the average number of years a person would live if they experienced the age-specific mortality rates for the population as a whole. We use published data to compare LE by local area with mortality rates by cause of death in categories with a proven link to smoking, such as deaths from lung cancer, to shine a light on health inequalities.
Health expectancies provide estimates of how long a person can expect to live disability free or in very good or good health. The two definitions that are widely used in the U.K. are HLE and disability-free LE (DFLE). HLE, the simpler of the two, is based on whether a person reports that their health is good, fair, bad or very bad. DFLE is based on whether health or disability problems affect one’s ability to carry out normal daily activities, such as household chores like washing, dressing, shopping (Office for National Statistics, Health state life expectancies, UK QMI, 2018). In the U.K., detailed estimates of LE, HLE and DFLE are produced by the ONS down to local authority level (Office for National Statistics, Health state life expectancy, all ages, UK, 2022).
Unlike LE, which is ultimately derived from data in the system for registering births and deaths, both HLE and DFLE are based on self-reported measures using data collected in the Annual Population Survey (APS). This is a large, repeated, cross-sectional survey of 320,000 households covering ages from 16 to 95, with the health status of those falling outside this age range estimated using imputation or other survey sources. Mortality and mid-year population data are aggregated over a 3-year period (2015–2017) to achieve a sufficient sample size required for the calculation of LEs by single year of age (SYOA) using the Chiang method (Chiang 1984). HLE and DFLE are calculated using the Sullivan method (Sullivan 1971) using 3 years of aggregated data incorporating LEs and survey health data.
Health expectancies are calculated for ages 18 and over. Data are split by smoking and economic activity identifiers and standard APS re-estimation methods are applied to obtain weighted counts of good health and disability. This means that the sample is large enough to allow us to analyse the health and work spans of different population segments according to gender, whether they smoke and if they are economically active. We use the information derived from the APS in support of all the analysis in the third section of this paper for comparing the health and disability status of economically active or inactive smokers and non-smokers by gender and other subgroups.Footnote 1
For example, we compare how many of the remaining years of life will be spent in good health using a life table approach by single year of adult age. A limitation is that we are unable to link the survey data to death registrations and so enable a direct relationship between life and health status of individuals, whilst separate life tables by smoking status do not exist. However, we can compare the burden of ill health at district level by analysing the gap between LE and HLE, which we use as a proxy for the average number of years spent in ill health.
Working life expectancy (WLE) is still a relatively new concept (Mayhew 2009; Mayhew 2021). It is defined as the expected number of economically active years spent between entering work and retirement. If we assume, for the sake of argument, a person starts work at age 20 and ceases work at age 65, then a person who is active for the whole of the period would have a working life of 45 years, but if they spend only half that time economically active their WLE would be 22.5 years.
We can extend this concept to LE and HLE, which we make conditional on a person attaining adulthood, in this case 20. For example, if the LE of a person aged 20 is 58 years, the person’s expected age of death is 78 years and similarly for HLE (and DFLE). By so doing, we are able to compare the WLE of people in the same units that we measure HLE, DFLE or LE. We can also split them further according to whether they are economically active or not, or never, current or ex-smokers using APS data.
Smoking and health
There are different ways to show the impact of smoking on heath and mortality. In this section, we draw a distinction between those that correlate with smoking prevalence and life or health expectancy, and those that correlate with cause of death such as lung cancer. Typically, analyses of inequalities focus on differences in LE, but we have broadened our investigation across multiple health and mortality metrics.
A key finding is that variations in LE and health expectancy are not only correlated as one would expect, but that variations in HLE are significantly greater than those in LE. Additionally, the gap between HLE and LE is highest in the areas of lowest LE and least in the areas of highest LE, indicating that not only are lives shorter but the proportion of life spent in ill health is greater.
In our second illustration, we use a life table approach to show the lifetime impact of smoking on health and work and the progressive negative impact of smoking on future health expectancy compared with never smokers. We show that smoking also impacts work capability and smokers have shorter working lives, and that the consequent loss of output and earnings comes at a huge economic cost.
The association of smoking with health and mortality at local area level
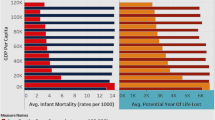
Figure 2 shows the variation in male life and health span measured at age 20, the onset of adulthood. In the left-hand panel, we compare life span across 150 English Upper Tier Local Authorities (UTLAs) with health span. Health span is estimated using APS cross-sectional data for each UTLA using published ONS tables. LE is separately calculated from UTLA life tables, both estimations using ONS methodology.
a Life expectancy for males aged 20–24 versus health expectancy by Upper Tier Local Authority (UTLA); b Map of England for males, showing health span by UTLA. Notes: ‘P’ denotes the lower end of the health expectancy scale and ‘Q’ the upper end. The vertical distance gives a measure of the years spent in ill health (see footnote 1). Health expectancy calculations make use of UTLA-specific life tables
We find that, on average, there is about a 17-year difference in health expectancy between the best and worst performing UTLAs and a 9-year difference in LE, indicating significant health inequalities between areas. It shows that life span and health span are highly correlated but that the increase in life span corresponding to a 1-year improvement in health expectancy is less than 1 year.
A best fit regression shows that a 1-year improvement in HLE increases LE on average by 4.5 months. This is important because it means that, as HLE increases, the gap between life span and health span gets smaller. Equivalently, a 1-year improvement in LE is associated with a 2.6-year increase in HLE. The predicted value of the gap varies from 21 years at point P at the bottom end of the HLE range to 12 years at point Q at the top end (with a high coefficient of determination, R2 = 0.74).
Since the size of the vertical gap equates to the average years spent in ill health, how this gap impacts on different areas of the country is potentially indicative of healthcare costs as well as fitness for work in those areas. This gives us an insight into not only the degree of inequality between areas but also how much the economy is impacted in terms of the cost measured in terms of healthcare and lost output from reduced labour force activity (Mayhew 2021).
The map of England in Fig. 2b shows how health span varies spatially by UTLA. It can be seen that the lowest health spans are concentrated mostly in cities (London, the Midlands and the North), but most noticeably in a band linking Liverpool, Manchester, Leeds and Hull.
Underlying these observations are particular medical conditions which can be linked to lifestyles or other factors. Smoking is a risk factor for many chronic diseases, some of which are later determined to be the main or contributory cause of death globally. Smoking is attributed to many deaths from respiratory diseases, cardiovascular diseases and cancer in England, with lung cancer being the most well known (Office for Health Improvement and Disparities 2023a).
To illustrate this, the maps in Fig. 3a and b show the pattern of male deaths from lung cancer in England per 100,000 between the ages of 20 and 89, also by UTLA, and the equivalent pattern for women. The similarity in patterns between male deaths from lung cancer and health expectancy in Fig. 2b is striking. Male deaths from lung cancer were higher in northern, Midlands and coastal cities in areas where health span is lower.
We note in passing that this pattern is similar to the pattern for deaths from heart disease, which is also implicated in smoking-related deaths, but for deaths from other types of cancers the geographical patterns are more dispersed. Since fewer women smoke than men the mortality rates from lung cancer are lower but there are similar concentrations of deaths in northwestern and northeastern cities.
The impact of smoking on health over the adult life course
Our second illustration takes a life course approach in which we show the negative impact of smoking on health at every age, as the following charts show. These consider the separate cases of never, current and ex-smokers. The data based on the APS, which includes questions about smoking behaviour and general health, were especially commissioned from ONS and can be accessed on request to ONS.
This analysis covers the whole U.K. and is broken by single year of age, gender and smoking status. However, the sample sizes are too small to break down by area as with the first illustration. In effect, our estimates of health expectancy are national averages, and so we do not observe the spread of variation between local areas, but we do have the advantage of being able to compare health expectancy by start age.
Figure 4 shows the value of HLE based on adults aged 20+ in three categories: all persons, never smokers, and current and ex-smokers. We observe that never smokers enjoy 48 years of healthy life at age 20 and current and ex-smokers only 42 years (all-person average 45 years). If we compare the all-person case with LE at age 20 of 61 years using ONS life tables, we find a 16-year gap between life and health span (81−65=16) which is only slightly higher than the 15-year predicted gap in Fig. 2a with reference to the x-axis for someone with a health span of 65 years.
We also note in passing that comparing our results with HLE by deprivation decile provides independent confirmation that health inequalities and deprivation are closely linked. The horizontal arrow in Fig. 4 highlights the age difference of 6 years between a smoker and never smoker with the same HLE, which can be interpreted as having the same biological age. Therefore, if never smokers had a biological age equal to their chronological age of 40 years, smokers aged 34 years would have a biological age of 40 years.
Figure 5 shows the difference in adult HLE between never smokers and current and ex-smokers in 1-year steps from age 20. The gap in health expectancy denoted by the vertical arrow is greatest between non-smokers and never smokers and is highest in young adults, by about 6 years. The gap closes with age with the remaining years of health declining to zero. Note that the spread in HLE values in Fig. 2a is rather wider than in Fig. 5 because it combines all risk factors and not just smoking, which varies substantially between UTLAs according to socioeconomic factors.
There are substantial differences between males and females. Figure 6 shows that never-smoking females enjoy up to six extra years of health than female smokers, slightly less than for males. This is because female smokers smoke less (cumulatively) than male smokers. For example, smoking is known to be harmful in pregnancy and to young children, which is an encouragement to give up smoking (Fig. 7).
Comparing alternative measures of health expectancy, we find that the impact on from smoking is less than the impact on health expectancy—equating to about 1 year at age 20. This suggests that activities of daily living may be less affected by smoking than physical health.
Our data also allow us to compare DFLE with the WLE of economically active adults who are never smokers with those of never smokers. Table 1a compares male DFLE at ages 20 to 70 in 10-year steps with WLE and economic activity rates.
It shows that male never smokers enjoy five more years of DFLE at the age of 20 than current/ex-smokers, and 2.5 more years of WLE. They are also more likely to be economically active at ages 30 and above, enjoy 3.4 more years of DFLE at the age of 50, and 1.6 more years of WLE at age 70. They are also 7.2% more likely to be economically active than current/ex-smokers at age 50.
Table 1b shows that female never smokers enjoy 5.8 more years of DFLE at the age of 20 than current/ex-smokers, 0.8 years more than men. They are also more likely to be economically active across ages 30–60, and experience 3.0 years of higher WLE at age 50 than current or ex-smokers. They are also 5.5% more likely to be economically active at age 50.
Since our sample is not linked to age of death, we are unable to provide estimates of LE alongside estimates of DFLE and WLE by smoking status and age, only an overall figure. The most we can say is there is a wider gap between DFLE and LE at each age in Table 1, with women experiencing more years with disability than men, albeit living longer. The average for both sexes of living with a disability ranges from 15.3 years at age 20 to 7.5 years at age 70.
The impact of smoking on economic activity at each age is seen in Fig. 8. This shows the percentage of economically active men by single year of age according to whether they are never smokers or current or ex-smokers. We observe that economic activity generally winds down after age 60, the rates tended to equalise, but those who had never smoked were still more likely to remain economically active into later life.
The biggest differences occur approximately from ages 30–60, where we find that never smokers have activity levels that are up to 20% higher. These differences could be due to poorer health, occupational or educational differences, or other factors among current and ex-smokers. Educational effects are likely indicated in the 20–25 age range: here, never smokers are economically less active but also more likely to be in full-time education.
Such differences have the potential to reduce economic output to the extent that working lives are shorter and lifetime earnings less. Assuming that annual average earnings in the U.K. were GBP 25,000 during this period, the International Longevity Centre U.K. has estimated that the difference in equivalent economic activity is such that if no men in the U.K. had ever smoked, the U.K.’s GDP would be GBP 11.5 billion higher per year (Mayhew and Dimitriadis 2021). The equivalent boost for women is GBP 7.6 billion, giving a total of GBP 19.1 billion in economic losses per annum. Note that a full economic cost-benefit analysis of the impact of smoking cessation would also take into account other costs—for example, sickness and disability benefits, healthcare costs and costs of formal and informal care.
Connectivity of smoking with other risk factors
Smoking does not exist in isolation from other risk factors, which may add to health risks and the probability of death. These risk factors may co-occur with smoking to different degrees. In some cases, other behavioural risk factors may reinforce smoking habits or they may have the opposite effect. In general, the reinforcing risk factors can be expected to make the achievement of a 5-year improvement in HLE harder.
Among the risk factors that tend to cluster together are smoking, nutrition, alcohol consumption and physical activity (abbreviated as ‘SNAP’). Table 2 shows the percentage of smokers exposed to each using data taken from an epidemiological study on the prevalence of SNAP risk factors in a representative sample of 3034 adults in the U.K. (Birch et al. 2018). Each row shows the number of people in each of eight categories who consume harmful levels of alcohol, are overweight or obese or are physically inactive. These are split into eight mutually exclusive groups, which include 804 people (group seven) that are not exposed to any of the risk factors.
The second-to-last column to the right shows the percentages of each risk group that are smokers, and the column totals the numbers exposed to each risk separately. The rows are ordered in terms of those at greatest risk of smoking—for example, the top category with 51 members or 1.68% of this population is at alcohol risk and physically inactive and, of these, 39.2% are smokers. The top four risk categories accounting for 16% of the sample are all at alcohol risk, of whom 23% smoke.
Overall smoking prevalence in this population is 15.2%, as shown in the bottom right of Table 2, which compares closely with smoking prevalence in the whole population. Other categories are generally less likely to be smokers than the average person, especially the obese or overweight. There may be a connection between the two risk factors—smoking is a known appetite suppressant and therefore an inverse relationship between smoking and putting on weight is to be expected.
We can predict the probability of smoking from combinations of these risk factors, using logistic regression. This shows that the odds of being a smoker increase 1.9 times among people who consume harmful levels of alcohol, 1.3 times among the physically inactive and 0.7 times among the overweight—so the effect of obesity on smoking is less than even. The risk factors are multiplicative so that somebody who drinks a lot and is physically inactive is 2.5 times more likely to be a smoker. The three factors together explain 85% of the differences in smoking behaviours between the eight risk groups (i.e. have a high coefficient of determination, R2 = 0.85).
These results suggest that, after smoking, obesity may be the next best risk factor to analyse in terms of its impact on HLE. In fact, a Health Survey for England study of multiple risk factors in a representative subset of the English population (the same four ‘SNAP’ factors and fruit and vegetable consumption) suggests similar priorities (Scholes 2018), beyond increasing fruit and vegetable consumption, where no dietary or public health interventions that tackle this have been considered (Ford et al. 2021).
Acting on obesity, which is also becoming a leading cause of death, also ties in with current public health policy priorities (although there was a recent policy U-turn in the U.K.). Even if obesity is a consequence of health behaviours rather than a medical condition, clustering and other data-driven analyses tend to be correlated; however, policy solutions show little overlap with smoking cessation except in the sense that lapsed smokers risk becoming overweight. The web of causation among health behaviours, socioeconomic circumstances and health outcomes is therefore complex (Wilson et al. 2006), and patterns and trajectories of multimorbidity (having more than one, perhaps interlinked risk factors) are difficult to analyse.
As a result, the effects of wider socioeconomic, cultural and environmental factors and their interactions with lifestyle factors are often qualitatively (but not usually quantitatively) discussed in public health reports (Dahlgren and Whitehead 1991). A rare quantitative study that assessed the lifelong impact of multiple risk factors compared a group of individuals aged 50+ years who never smoked, were not obese and consumed alcohol moderately in the U.S. to the general U.S. population, and found that their LE at age 50 was up to 7 years longer, while their DFLE at age 50 was up to 6 years longer (Mehta 2017).
The causes of ill health are increasingly lifestyle related and rooted in the cultures of different socioeconomic groups—these include smoking, excessive drinking, drug abuse, mental illness and obesity (Chandola et al, 2004). However, we should not approach this patterning of health with a fatalistic lens but rather be more proactive (Marmot et al. 2010). A nationwide analysis of cancer data in the U.S. found that 47.9% of deaths from cancer were attributable to avoidable risk factors including cigarettes (33.1%), excess body weight (5.7%) alcohol (4.3%) and other factors such as little physical exercise and diet (4.8%) (Islami, et al., 2018).
An alternative approach to assessing the impact of smoking cessation
The discussion so far represents one approach to assessing the impact of smoking cessation. However, this side steps the fact that there is no ideal dataset that charts the lives of individuals, including smoker status throughout their life course, relevant socioeconomic factors or life events and, ultimately, date and cause of death. Another approach, still under development, is to use cause-of-death data. Provided such data have sufficient granularity, we can focus on significant causes of death where smoking is the main risk factor.
For smoking, the key diseases are lung cancer and chronic obstructive pulmonary disease (COPD). The use of lung cancer and COPD mortality data allows us to make inferences about relative levels of smoking in different areas: if lung cancer death rates are twice as high in one group as another, then smoking prevalenceFootnote 2 is approximately twice as high. One of the challenges is to see if one can work backwards from cause and age of death, which are widely available from registration systems, to determine the average years spent in good health.
The model used here (the common cohort effects model, CCE; Cairns 2022) assesses relative levels of smoking between cohorts in a given population: see the Appendix for a more-detailed description of how the model works. The model identifies distinct cohort effects for smoking and other controllable risk factors such as excessive alcohol consumption. It allows us to address questions such as what the impact would have been on death rates if smoking prevalence had been 20% lower (in relative terms) in each cohort. It finds that death rates from lung cancer and COPD would have each been approximately 20% lower given but other causes of death which have smoking as a risk factor would have been lower as well. The CCE model allows us to quantify this impact.
The approach has been applied to a U.S. dataset (Redondo Loures and Cairns 2021) with results reported in Table 3.Footnote 3 The table suggests that for high- and low-educated U.S. males and females, a 20% reduction in smoking prevalence would have resulted in all-cause death rates being between 8 and 12% lower for low-educated males (who, historically, have had the highest levels of smoking) and between 2 and 6% lower for high-educated females (lowest smoking prevalence).
It is important to note that in this scenario we are attempting to assess what death rates would be like if historical levels of smoking had been 20% lower than they were in fact. This is different from the alternative scenario in which 20% of current smokers quit permanently. In this case, there would be a gradual decline in death rates to levels in the first scenario, but not until well after the government’s target of 2035. This gives a truer picture of the potential impact on mortality of smoking cessation in the long run, which is of a steady decline rather than an abrupt reduction.
Typically, a 10% reduction in the all-cause death rate equates approximately to a 1-year increase in remaining LE at younger ages for the whole cohort.Footnote 4 The results suggest, therefore, that a 20% reduction in smoking prevalence equates to around a 1.2-year improvement in LE for young, low-educated males, but only 0.24 years for young, high-educated females.
For current purposes, we are interested in estimating the corresponding impact on HLE. The CCE model only allows calculation of LE, so some assumptions are required to calculate HLE. We can appeal to the relationship between LE and HLE in Fig. 2a. The linear relationship revealed there suggests that one additional year of LE equates to 2.6 years of additional HLE.
It might be that a 1-year gain due to reductions in smoking prevalence has a different impact on HLE than a 1-year gain due to reductions in excessive alcohol consumption or other risk factors but, as far as we are aware, data are not available to assess these differences. So, we make an assumption that the impact on HLE of a 1-year reduction in LE is the same for all major risk groups. If correct, then an improvement in LE would lead to a significantly higher proportion of the population in good health.
This provides a benchmark for future studies that would aim to assess, more directly, the improvement in HLE as a result of reduced numbers of deaths from specific diseases. For example, for a typical person (a smoker) dying at age 75 from COPD, how many years did that person spend in poor health? How does that compare with someone aged 75 dying from alcohol-related liver disease or diabetes?
Of what significance are these U.S. results to potential smoking policies England? In England, higher levels of smoking (as inferred from lung cancer death rates, Fig. 3) are strongly correlated with low health spans (Fig. 2). It follows that a successful and effective campaign focussed on smoking cessation in areas with currently high levels of smoking will, in the long run, go a long way towards reducing health inequalities but the results would take time to work through.
What can we learn from research and policies in the U.K. and other countries?
A range of policies and interventions have been attempted with the aim of reducing smoking prevalence and initial uptake: some universal and others targeted at specific subgroups. Many types of these policies have already been introduced in the U.K. (Fig. 1), although not as stringently as in some other countries, such as bans on smoking in public places.
The U.K. Public Health Grant has funded a wide range of public health and therapeutic interventions (Ford et al. 2021)—many of which were efficacious, effective and affordable globally (West et al. 2015); all of the 67 interventions studied in England were found to be cost-effective. Taxation, usually through smoking duties, is a commonly used policy which has been found to have mixed effects on widening or narrowing inequalities (Blakeley 2019).
Lastly, a complete ban on smoking may not be unrealistic as more drastic policies on smoking cessation are currently being implemented in some countries. For example, in New Zealand, a strict cohort-specific tobacco ban was planned from July 2024 for New Zealanders born after 1st January 2009, although the act has just been repealed by the new Government (CNA 2023), whilst smoking in all indoor workplaces, bars and hospitality venues has been outlawed since 2004. Other countries like Singapore, Malaysia and Denmark are considering following suit (CNA 2023).
Several modelling approaches have been used to investigate the potential impacts of smoking interventions that fall short of a complete ban. In health economics, more realistic decision analytic models have been used. Discrete choice experiments (Buckell et al. 2020; Levy et al. 2017) have been used to factor in and simulate individuals’ behaviours in choosing between tobacco types and the resulting impact on mortality.
In particular, a simulation study of smoking cessation in the U.S. analysed the impact of (1) increased quit attempts, (2) treatment use and (3) treatment effectiveness. Prevalence was projected to reduce from 20 to 12% over 4 years assuming an extreme scenario of 100% effectiveness for all three (Levy et al. 2010). Only when these extreme assumptions are applied do we see a reduction in prevalence in this study on the scale of the desired reduction for the U.K. to be smoke free by 2030 (from 14% in 2020 to a potential level of 0% in 10 years; Fig. 1).
In biostatistics and epidemiology, more advanced statistical methods (e.g. multi-state life tables and Markov multi-state models) and more objective definitions of the onset of poor health (e.g. recorded medical diagnoses of diseases) have been used to analyse HLE patterns by socioeconomic status and by smoking status, using individual-level data e.g. a study of socioeconomic inequalities in HLEs of 1.1 million older English adults (Chan et al. 2019).
This study found that current and ex-smokers spent similar durations of their remaining life healthy, and less time than never smokers. However, this particular study utilised the onset of multimorbidity to define entry into the ill-health state in HLE estimation, rather than the ONS definition of self-reported ill health. A similar study investigated the interplay between HLE and WLE and estimated that healthy working LE (HWLE) at age 50 in England of 9.42 years was below the current State Pension Age (Parker et al. 2020).
Discussion
Key findings and implications
The negative impacts of smoking on disease and cause of death are well documented but the impact on health much less so, except in some obvious cases where certain diseases are directly attributable (Islami et al, 2018). Much less is known about the impact on HLE generally, on the wider economy or the indirect effects such as caring for people with smoking-related disease.
This issue has been brought to the fore by the U.K. Government’s target of wanting to raise U.K. HLE by 5 years by 2035. It raises the obvious question of whether it is feasible or not and, if so, what steps are needed to make it happen. Since smoking is still a leading cause of death resulting in premature mortality, it logically follows that it is also one of the main causes of ill health.
Despite welcome falls in smoking prevalence over recent decades, we are not out of the woods and there is huge geographic and socioeconomic variation. Although never smokers stand to gain 6 years of health expectancy at age 20 relative to current and ex-smokers, our results show that the impact of smoking cessation will not be immediate due to the health impacts of previous smoking.
In this paper, we investigated the degree to which health expectancy would be boosted by a complete cessation of smoking and if it would be enough to meet the U.K. Government’s target. As smoking prevalence has already been lowered in recent decades, we found that potential advancement is only around 2.5 years rather than the full 6 years if everybody in the population was a smoker.
Our analysis also demonstrated that this impact depends on the measure used: DFLE increased by 5–5.8 years while WLE increased by 1.8–2.5 years if people aged 20 who were smokers were non-smokers instead. This corresponds to all (DFLE) or half (WLE) of the HLE + 5 target. It also depends on the ages at which cessation occurs: the smokers versus non-smokers gap narrows to 1.2–1.6 years (DFLE) or 0–0.6 years (WLE) at age 70, although this narrowing captures survival bias.
If smoking prevalence had been 20% lower, lung cancer mortality would reduce by 20% and COPD mortality by 18–22%. This is a large 1-to-1 impact on mortality, but still needs to be translated into HLE impacts. This implies that only a small fraction of these increases could materialise, as currently only 14% of the U.K. population are smokers (Fig. 1), and smoking cessation interventions are not 100% effective.
However, we also saw that in the areas most at risk, the potential for improvement is much higher. We found, for example, a strong link between shorter HLE and deaths from lung cancer, especially for men who smoke more than women, and also more years spent in ill health towards the end of life. But even if all smoking ceased tomorrow, the whole process could take decades to work though.
We conclude that drastic smoking cessation intervention is necessary to increase HLE and narrow the inequality gap in HLE—for example, a total ban on smoking could be an important first step. If implemented in the next decade, the countries that implement this ban will have strong precedents to inform this implementation in the U.K. or other countries that follow suit.
From an economic point of view, that a ban could extend working lives is confirmed in other research (Parker et al. 2020), thereby increasing productivity and more than recouping the loss of tobacco duties. A complete ban would enable more people to reach the higher state pension age planned for the next decades both alive and in good health. It is estimated that annual savings to the NHS could be GBP 2.4 billion (Office for Health Improvement and Disparities 2022), which does not include the wider work-related economic benefits identified earlier (Office for Health Improvement and Disparities 2022).
Findings from other countries and modelling studies currently do not point towards a clear action plan for meeting the HLE targets via smoking cessation. Other interconnected risk factors suggest the use of joined-up approaches for both intervening and modelling and would contribute further to identifying and targeting higher risk population subgroups and areas. However, voluntary methods of reducing smoking prevalence short of a ban are generally currently in use and seem unlikely to make a big difference unless backed up by tighter controls and restrictions.
Conclusion
Despite the strength of our findings, our results do not address the practicalities of what is achievable over the timescale to be ‘Smoke free by 2030’, which was an initiative commissioned by the U.K. government, or how this would contribute to the U.K. government’s wider levelling up agenda. For example, there are gaps in our knowledge of the effectiveness of smoking cessation programmes and which work best and in what circumstances, and indeed whether there is sufficient funding to sustain current programmes, but also what adjustments would be needed to these services in the event of a total ban.
In summary, the U.K. population continues to age, and consequently, grow increasingly unhealthy regardless of whether it smokes or not, and so it will become more important for policymakers to support longer working lives and spend more on preventative healthcare. Combined, these measures would both support the economy and prevent millions falling into poverty (Office for Health Improvement and Disparities 2022). This study adds to the evidence base for policymakers to take action on smoking—the local areas that would benefit most from intervention were identified and the relative health benefit of targeting younger people (who are also unlikely to have developed smoking habits) have been demonstrated.
Our findings suggest that smoking reduces HLE and increases disability: smokers and ex-smokers can expect to live substantially fewer years in good health and without disability than non-smokers. In terms of wider implications, reducing the impact of smoking on the health of the U.K. could benefit the economy by up to GBP 19 billion a year and reduce the burden on and cost to the NHS and welfare system (Mayhew and Dimitriadis 2021). For these reasons, we support stronger measures like banning the sale of tobacco, following a measured approach such has been discussed New Zealand. Looking at tobacco control data published by the World Health Organization (WHO 2023) is a reminder that tobacco consumption globally varies widely and that outright bans may not be the right answer in every case; other health priorities may be more important and a more gradual approach may be warranted. We also think that our analysis may have implications for the wider pensions and insurance industry in terms of product pricing, although we do not pursue these connections here.
Are there wider lessons to be learned?
The 5-year target to improve health expectancy by 5 years has been useful in helping us to focus our research, even though we found that, by itself, a complete smoking ban would be insufficient. The target itself remains an important rallying flag, especially if further research is able to evidence other policies that would be as effective. However, there is an ever-present danger of it becoming discredited if progress is slow or even goes into reverse—this is why stronger measures are required. As Charles Goodhart notes: “When a measure becomes a target it ceases to be a good target” (Goodhart’s Law).
Notes
Sources of data on health expectancy can be found at Health state life expectancy, all ages, UK—Office for National Statistics (ons.gov.uk).
Strictly speaking, smoking prevalence is just one factor that influences lung cancer death rates. Lung cancer death rates also depend on smoking intensity, time since commencement and patterns of smoking cessation. We use the term “smoking prevalence” here as a proxy for the combination of these factors and their impact on death rates.
At present, data for England lack the required level of granularity to make it possible to fit the CCE model.
That is, if underlying death rates follow a Gompertz model with a growth rate of 0.1, which is a reasonable approximation for a range of populations from ages 40 onwards.
References
Binns, R. 2018. Fairness in machine learning: Lessons from political philosophy. Proceedings of Machine Learning Research 81: 1–11.
Birch, J., R. Petty, L. Hooper, L. Bauld, G. Rosenberg, and J. Vohra. 2018. Clustering of behavioural risk factors for health in UK adults in 2016: A cross-sectional survey. Journal of Public Health 41: e226–e236.
Blakely, T., and C. Gartner. 2019. Tobacco taxes have mixed effects on socioeconomic disparities. The Lancet Public Health 4: e595–e596.
Buckell, J., Hensher D. A. and S. Hess 2020. Kicking the habit is hard: A hybrid choice model investigation into the roles of addiction in soking behaviour. Health Economics.
Cairns, A.J.G. 2022. Cohort effects in US cause-of-death mortality data: The link to controllable risk factors. Presentation at the human mortality database conference, Paris, October 2022. Paris. www.macs.hw.ac.uk/~andrewc/ARCresources/Cairns_HMD2022.pdf
Chiang, C.L. 1984. The life table and its applications. Malabar, FL: Robert E. Krieger Publishing.
Chan, M. S., A. van den Hout, M. Pujades-Rodriguez, M. Jones, F. Matthews, C. Jagger, and M. Bajekal. 2019. Socio-economic inequalities in life expectancy of older adults with and without multimorbidity: A record linkage study of 1.1 million people. International Journal of Epidemiology 48: 1340–1351.
Chandola, T., J. Head, and M. Bartley. 2004. Socio-demographic predictors of quitting smoking: How important are household factors? Addiction 99: 770–7.
CNA. 2023. IN FOCUS: Snuffing out smoking - is this the last mile in Singapore’s fight against tobacco? https://www.channelnewsasia.com/singapore/quit-smoking-tobacco-tax-hike-ban-vaping-addiction-3295746
Dahlgren, G., and M. Whitehead. 1991. Policies and strategies to promote social equity in health. Stockholm: Oxford Academic Press.
Department for Levelling Up Housing and Communities. 2022. Policy paper: Levelling up the United Kingdom. https://www.gov.uk/government/publications/levelling-up-the-united-kingdom
Development Strategy and Policy Analysis Division United Nations. 2015. Concepts of inequality: Development issues no. 1. https://www.un.org/en/development/desa/policy/wess/wess_dev_issues/dsp_policy_01.pdf
Doll, R., and A. Hill. 1950. Smoking and carcinoma of the lung; preliminary report. BMJ 2: 739–48.
Doll, R., R. Peto, J. Boreham, and I. Sutherland. 2004. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 328: 1519.
Everest, G., L. Marshall, C. Fraser, and A. Briggs. 2022. Addressing the leading risk factors for ill health. London: The Health Foundation.
Ford, J., N. Ekeke, and A.K. Lahiri. 2021. Making the case for prevention. Cambridge: University of Cambridge for the Health Foundation.
Green, D., G. Filkin, and T. Woods. 2021. Our unhealthy nation. The Lancet Healthy Longevity 2 (1): e8–e9.
Islami, F., A. Goding Sauer, K. Miller, R. Siegel, S. Fedewa, E. Jacobs, A. Jemal, et al. 2018. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA: A Cancer Journal for Clinicians 68: 31–54.
Levy, D.T., R. Borland, A. C. Villanti, R. Niaura, Z. Yuan, Y. Zhang, R. Meza, T. R. Holford, G. T. Fong, K. M. Cummings, and D. B. Abrams. 2017. The application of a decision-theoretic model to estimate the public health impact of vaporized nicotine product initiation in the United States. Global Public Health: 19:(2) 149-159.
Marmot, M., P. Goldblatt, and J. Allen. 2010. Fair society, healthy lives: the marmot review: Strategic review of health inequalities in England post-2010. London.
Marteau, T.M., M. White, H. Rutter, M. Pettigrew, O.T. Mytton, J.G. McGowan, and R.W. Aldridge. 2019. Increasing healthy life expectancy equitably in england by five years by 2035: Could it be achieved? The Lancet 393: 2571–2573.
Mayhew, L. 2009. Increasing longevity and the economic value of healthy ageing an working longer. London: Cass Business School, commissioned report by the Cabinet Office Strategy Unit.
Mayhew, L. 2021. The cost of inequality—Putting a price on health special report by the International Longevity Centre UK, CSFI and Cass Business School. London: International Longevity Centre UK, CSFI and Cass Business School.
Mayhew, L., and S. Dimitriadis. 2021. Up in smoke: The impact of smoking on health and economic activity. London: International Longevity Centre UK.
Mehta, N., and M. Myrskylä. 2017. The population health benefits of a healthy lifestyle: Life expectancy increased and onset of disability delayed. Health Affairs 36: 1495–1502.
NHS England. 2023. What are healthcare inequalities? https://www.england.nhs.uk/about/equality/equality-hub/national-healthcare-inequalities-improvement-programme/what-are-healthcare-inequalities/
Office for Health Improvement and Disparities. 2022. Independent report: The Khan review: making smoking obsolete. https://www.gov.uk/government/publications/the-khan-review-making-smoking-obsolete
Office for Health Improvement and Disparities. 2023a. Fingertips public health data: Local tobacco control profiles. https://fingertips.phe.org.uk/profile/tobacco-control/data#page/0/gid/1938132887/pat/6/par/E12000001/ati/302/are/E06000047/cid/4/tbm/1
Office for Health Improvement and Disparities. 2023b. Guidance: Smoking and tobacco: Applying all our health. https://www.gov.uk/government/publications/smoking-and-tobacco-applying-all-our-health/smoking-and-tobacco-applying-all-our-health
Parker, M., M. Bucknall, C. Jagger, and R. Wilkie. 2020. Population-based estimates of healthy working life expectancy in England at age 50 years: Analysis of data from the English longitudinal study of ageing. Lancet Public Health 5: e395–e403.
Public Services Committee. 2020. A critical juncture for public services: Lessons from COVID-19. https://committees.parliament.uk/publications/3438/documents/32865/default/
Redondo Loures, C., and A.J.G. Cairns. 2021. Cause of death specific cohort effects in U.S. mortality. Insurance: Mathematics and Economics 99: 190–199.
Scholes S. 2018. Health survey for england. Mulitple risk factors. London.
Sullivan, D.F. 1971. A single index of mortality and morbidity. Health Services Mental Health Administration Health Reports 86: 347–354.
West, R., M. Raw, A. McNeill, L. Stead, P. Aveyard, J. Bitton, R. Borland, et al. 2015. Health-care interventions to promote and assist tobacco cessation: A review of efficacy, effectiveness and affordability for use in national guideline development. Addiction 110: 1388–403.
World Health Organization. 2023. WHO report on the global tobacco epidemic, 2023: Protect people from tobacco smoke. https://www.who.int/publications/i/item/9789240077164
Wilson, N., T. Blakely, and M. Tobias. 2006. What potential has tobacco control for reducing health inequalities? The New Zealand situation. International Journal for Equity in Health 5: 14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
MSC is employed by Health Analytics, Lane Clark & Peacock LLP and the views expressed in the article are the author's own. All authors declare that they have no known competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: The Common Cohort Effects (CCE) model
Appendix: The Common Cohort Effects (CCE) model
This Appendix details the CCE model of Cairns (2022). The model for cause-specific death rates is as follows:
where c is the cause of death, t is the calendar year, x is the age and t − x is the year of birth. \(\alpha \left(c,x\right)\) is a non-parametric age effect. The \({\beta }_{k}\left(x\right)\) are pre-specified parametric functions of age: in this case, constant, linear and quadratic. The \({\kappa }_{k}\left(c,t\right)\) are cause-specific period effects. This model applies the CBD-X3 model of Dowd et al. (2020) for all-cause mortality to a new setting involving multiple causes of death.
In this model, the \({\chi }_{j}(t-x)\) are the n common cohort effects and n is typically quite small, e.g. n = 3. Finally, the \({\delta }_{j}\left(c\right)\) specify the impact of common cohort effect \({\chi }_{j}\left(t-x\right)\) on cause of death c. The underlying idea is that the common cohort effects capture the extent of controllable risk factors such as smoking (j = 1) or excessive alcohol consumption (j = 2) that are known to have a significant effect on certain causes of death. For example, suppose \({\chi }_{1}\left(t-x\right)\) is the smoking-related cohort effect, then \({\delta }_{1}\left(c\right)\) will be relatively large for diseases such as lung cancer and COPD with a known, strong link to smoking, and close to zero for causes where smoking is not known to be a significant risk factor.
The model has been fitted to U.S. mortality data by cause of death (51 individual causes) for males and females and by education level, with parameters estimated using maximum likelihood and optimisation simultaneously over all individual causes of death. To capture the scenarios reported in the fourth section, we reduce the smoking-related common cohort effect \({\chi }_{1}\left(t-x\right)\) by a constant amount across all cohorts and in a way that lowers lung cancer mortality by exactly 20%.
The model then tells us what the impact would be on all other causes of death. For COPD, there is also a reduction of about 20% in death rates, but, for other causes of death, the reduction in the death rate is mostly much smaller as smoking is much less significant as a risk factor. The resulting death rates for all of the individual causes of death can then be added up to assess the impact on the all-cause death rate.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mayhew, L., Chan, M.S. & Cairns, A.J.G. The great health challenge: levelling up the U.K.. Geneva Pap Risk Insur Issues Pract 49, 270–294 (2024). https://doi.org/10.1057/s41288-024-00317-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1057/s41288-024-00317-0