Abstract
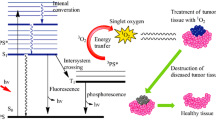
We recently introduced the concept of photodynamic molecular beacons (PMB) for selective control of photodynamic therapy (PDT). The PMB consists of a peptide linker that is sequence specific to a cancer-associated protease. A photosensitizer (PS) and a singlet oxygen (1O2) quencher are conjugated to the opposite ends of this linker. Proximity of the PS and quencher can efficiently inhibit 1O2 generation. In the presence of a targeted protease, the substrate sequence is cleaved and the PS and quencher will separate so that the PS can be photo-activated. There are two ways to optimize the PMB selectivity to cancer cells. The first is to increase the protease specificity to targeted cells and the second is to minimize the phototoxicity of intact (uncleaved) PMBs in non-targeted (normal) cells. Carotenoids (CARs) are well known in nature for their role in quenching excited states of PS and in directly scavenging 1O2. The purpose of this study is to evaluate whether the CAR with dual quenching modes (PS excited states deactivation and 1O2 scavenging) can be used to minimize the photodamage of intact PMBs to non-targeted cells. Thus, we synthesized a beacon (PPC) with a caspase-3 cleavable peptide linking a PS and a CAR quencher. It was confirmed that CAR deactivates the PS excited states and also directly scavenges 1O2. Moreover, the in vitro PDT response showed that CAR completely shuts off the photodynamic effect in non-targeted HepG2 cells, while PS without CAR (control) remains highly potent even at a much lower (30-fold) dose.
Similar content being viewed by others
References
T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan, Q. Peng, Photodynamic therapy, J. Natl. Cancer Inst., 1998, 90, 889–905.
B. C. Wilson, M. S. Patterson, The physics of photodynamic therapy, Phys. Med. Biol., 1986, 31, 327–360.
J. Chen, K. Stefflova, M. J. Niedre, B. C. Wilson, B. Chance, J. D. Glickson, G. Zheng, Protease-triggered photosensitizing beacon based on singlet oxygen quenching and activation, J. Am. Chem. Soc., 2004, 126, 11450–11451.
G. Zheng, J. Chen, K. Stefflova, M. Jarvi, H. Li, B. C. Wilson, Photodynamic molecular beacon as an activatable photosensitizer based on protease-controlled singlet oxygen quenching and activation, Proc. Natl. Acad. Sci. USA, 2007, 104, 8989–8994.
R. Edge, D. J. McGarvey, T. G. Truscott, The carotenoids as anti-oxidants - a review, J. Photochem. Photobiol., B, 1997, 41, 189–200.
C. S. Foote, Y. C. Chang, R. W. Denny, Chemistry of singlet oxygen. XI. Cis–trans, isomerization of carotenoids by singlet oxygen and a probable quenching mechanism, J. Am. Chem. Soc., 1970, 92, 5218–5219.
A. Telfer, S. Dhami, S. M. Bishop, D. Phillips, J. Barber, beta-Carotene quenches singlet oxygen formed by isolated photosystem II reaction centers, Biochemistry, 1994, 33, 14469–14474.
A. J. Young, H. A. Frank, J. Photochem. Photobiol., B, 1996, 36, 3–15.
A. L. Moore, A. Joy, R. Tom, D. Gust, T. A. Moore, R. A. Bensasson, E. J. Land, Photoprotection by Carotenoids during Photosynthesis: Motional Dependence of Intramolecular Energy Transfer, Science, 1982, 216, 982–984.
E. Reddi, A. Segalla, G. Jori, P. K. Kerrigan, P. A. Liddell, A. L. Moore, T. A. Moore, D. Gust, Carotenoporphyrins as selective photodiagnostic agents for tumours, Br. J. Cancer, 1994, 69, 40–45.
M. Gurfinkel, A. B. Thompson, W. Ralston, T. L. Troy, A. L. Moore, T. A. Moore, J. D. Gust, D. Tatman, J. S. Reynolds, B. Muggenburg, K. Nikula, R. Pandey, R. H. Mayer, D. J. Hawrysz, E. M. Sevick-Muraca, Pharmacokinetics of ICG and HPPH-car for the detection of normal and tumor tissue using fluorescence, near-infrared reflectance imaging: a case study, Photochem. Photobiol., 2000, 72, 94–102.
D. Tatman, P. A. Liddell, T. A. Moore, D. Gust, A. L. Moore, Carotenohematoporphyrins as tumor-imaging dyes. Synthesis and in vitro photophysical characterization, Photochem. Photobiol., 1998, 68, 459–466.
N. A. Thornberry, Y. Lazebnik, Caspases: enemies within, Science, 1998, 281, 1312–1316.
W. Pham, R. Weissleder, C. H. Tung, An azulene dimer as a near-infrared quencher, Angew. Chem., Int. Ed. Engl., 2002, 41, 3659–3662.
K. Stefflova, J. Chen, D. Marotta, H. Li, G. Zheng, Photodynamic therapy agent with a built-in apoptosis sensor for evaluating its own therapeutic outcome in situ, J. Med. Chem., 2006, 49, 3850–3856.
Y. Choi, R. Weissleder, C. H. Tung, Selective antitumor effect of novel protease-mediated photodynamic agent, Cancer Res., 2006, 66, 7225–7229.
D. Gabriel, M. A. Campo, R. Gurny, N. Lange, Tailoring protease-sensitive photodynamic agents to specific disease-associated enzymes, Bioconjug. Chem., 2007, 18, 1070–1077.
M. J. Niedre, A. J. Secord, M. S. Patterson, B. C. Wilson, In vitro tests of the validity of singlet oxygen luminescence measurements as a dose metric in photodynamic therapy, Cancer Res., 2003, 63, 7986–7994.
M. Niedre, M. S. Patterson, B. C. Wilson, Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo, Photochem. Photobiol., 2002, 75, 382–391.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Jarvi, M., Lo, PC. et al. Using the singlet oxygen scavenging property of carotenoid in photodynamic molecular beacons to minimize photodamage to non-targeted cells. Photochem Photobiol Sci 6, 1311–1317 (2007). https://doi.org/10.1039/b706820d
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b706820d