Abstract
X-linked ocular albinism (OA1) is an X-linked inherited disease characterized by hypopigmentation of the fundus and nystagmus. Our study performed mutation analysis of the G protein-coupled receptor 143 gene (GPR143) and assessed the clinical characteristics of OA1 in three Chinese families. Three novel mutations, c.333_360+14del42insCTT, c.276G>A (p.W92X), and c.793C>T (p.R265X), were identified in GPR143 by PCR followed by Sanger sequencing in these families. All affected individuals presented with nystagmus, photophobia, poor visual acuity, foveal hypoplasia and varying degrees of hypopigmentation of the fundus. The fundus of female carriers showed pigmented streaks alternating with hypopigmented streaks. These results allowed us to expand the spectrum of mutations in GPR143 and phenotypes associated with ocular albinism.
Similar content being viewed by others
Introduction
Albinism consists of a rare group of clinical and genetic abnormalities caused by defects in melanin synthesis involving the hair, skin, and eyes. There are two main subtypes of albinism: oculocutaneous albinism and ocular albinism. The birth prevalence of X-linked ocular albinism (OA1; MIM 300500) is approximately 1 in 50,000. OA1 occurs almost exclusively in males, and results in only ocular abnormalities, including impaired visual acuity, nystagmus, photophobia, foveal hypoplasia, hypopigmentation of iris and fundus1. The fundus of female asymptomatic carriers exhibits a mottled pattern of pigmentation, which is special characteristic for OA1.
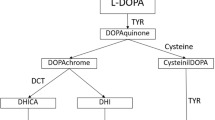
OA1 is caused by mutations in the G protein-coupled receptor 143 gene (GPR143) (OMIM 300808), originally called OA1, which is located at Xp22.322. GPR143 encodes a protein that binds to heterotrimeric G proteins and is highly expressed in melanocytes and the retinal pigment epithelium (RPE)2. It encodes a 404 amino acid protein predicted to be a membrane protein essential for development and maturation of melanosomes3. Unlike other GPCRs, GPR143 is not a transmembrane protein on the cell surface, but is exclusively localized to intracellular organelles, namely lysosomes and melanosomes4. It has been postulated that ocular albinism is a disorder of melanin secretion from the melanosome into keratocytes rather than a deficit in melanin synthesis. Mutant protein may lead to failure of melanosomes to bud off the endoplasmic reticulum. Instead, melanosomes are likely to aggregate as melanin accumulates, leading to the formation of giant melanosomes5,6.
Various types of mutations in GPR143 have been identified in patients from different countries; however, the characteristics of OA1 have not been well defined in Asians. X-linked OA1 in the Chinese population has been rarely reported7,8,9,10,11,12,13,14,15. As iris and fundus hypopigmentation is not obvious among the Chinese patients, it is difficult to distinguish OA1 from other congenital eye diseases, such as Leber congenital amaurosis (LCA), achromatopsia or congenital motor nystagmus (CMN). Children are always uncooperative for detailed retinal structure and function evaluation. In addition, Lack of awareness of the clinical features of ocular albinism by health care providers leads to frequent misdiagnosis. In this study, we describe the gene mutation and clinical manifestations of OA1 patients and carriers from three unrelated Chinese families. Our data expands the spectrum of phenotypes and mutations in the GPR143 gene in this population.
Methods
Recruitment of subjects
All participants were identified at the Ophthalmic Genetics Clinic at Peking Union Medical College Hospital (PUMCH), Beijing, China. Written informed consent was obtained either from the participating individuals or their guardians. This study was approved by the Institutional Review Board of PUMCH and adhered to the tenets of the Declaration of Helsinki and the Guidance on Sample Collection of Human Genetic Diseases by the Ministry of Public Health of China.
Clinical evaluations
A full medical and family history was taken. Patients underwent a detailed ophthalmic examination, including best-corrected visual acuity (BCVA) according to decimal E charts, slit-lamp biomicroscopy, dilated indirect ophthalmoscopy and fundus photography. The retinal structure was examined by optical coherence tomography (OCT, Topcon, Tokyo, Japan and Optovue Inc., Fremont, CA) and fundus autofluorescence (FAF, Heidelberg Engineering, Heidelberg, Germany). Electrophysiological assessment (Roland Consult, Wiesbaden, Germany) included a full-field electroretinogram (ERG) to test the entire retina and multi-channel visual evoked potential (VEP) to identify possible abnormal optic nerve decussation. The test protocols conformed to the standards of the International Society for Clinical Electrophysiology of Vision (www.iscev.org).
Genetic studies
Genomic DNA was isolated from peripheral leukocytes using the QIAamp DNA Blood Midi Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. GPR143 exons and adjacent sequences were amplified by the polymerase chain reaction (PCR) using primers published previously7. After purification, amplicons were sequenced using forward and reverse primers on an ABI 3730 Genetic Analyzer (ABI, Foster City, CA). Sequences were assembled and analyzed with Lasergene SeqMan software (DNASTAR, Madison, WI). The results were compared with the GPR143 reference sequence (NM_000273.2). All available family members were Sanger sequenced in order to confirm the segregation of mutations.
Results
Clinical phenotype
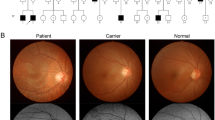
Three families diagnosed with OA1 were recruited for this study (Fig. 1). The clinical characteristics of OA1 in each affected individual are described in Table 1 and Fig. 2. Table 1 shows the gender, age and clinical characteristics of three patients and one carrier. The fundus images of the three probands, F1-II:1, F2-II:1 and F3-III:3 are shown in Fig. 2. Figure 3 shows the fundus image of a carrier, F1-I:2.
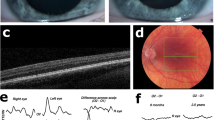
All OA1 patients in this study presented with nystagmus, photophobia and poor visual acuity. Nystagmus was present from birth, and BCVA was between Light Perception (LP) and 0.15. No BCVA data was obtained for Family 1 because the patient would not cooperate with the examination. The VEP and full field ERG were recorded from the proband of Family 3. All ERG waveforms were within normal limits; in particular, the amplitude of scotopic b-wave was close to the upper limit of normal, which is typical for albinism. The VEP showed a decreased amplitude of the P100 with normal latency for three channels. They were no differences among the three channels for either eye.
Only the patient from Family 3 had mild iris hypopigmentation. The iris of the other two patients appeared to be normal by slit lamp examination. All three patients exhibited hypopigmentation of the fundus, and all the patients had severe foveal hypoplasia (Fig. 2).
The carrier did not have any symptoms and her BCVA was normal (Table 1). The carrier exhibited normal iris pigmentation; however, the midperiphery of the fundus had alternating streaks that were hypopigmented or normally pigmented, illustrating lyonization, which is more obvious on FAF (Fig. 3). All the participants had normal skin and hair color.
Mutation analysis
Sequence analysis of GPR143 detected three novel mutations in the three families (Table 2, Fig. 4), c.333_360+14del42insCTT, c.276G>A (p.W92X), and c.793C>T (p.R265X), that are predicted to result in loss of function (LOF). These mutations were present in affected males as hemizygous and were heterozygous in female carriers.
Discussion
In our study, we identified three novel, predicted LOF mutations in GPR143 among three patients with OA1. All patients presented with severely impaired visual acuity, nystagmus, photophobia, foveal hypoplasia, and fundus hypopigmentation.
Since GPR143 is the only candidate gene for non-syndromic X-linked ocular albinism, we used Sanger sequencing to confirm the underlying gene mutation. Compared to next generation sequencing (NGS) and whole exome sequencing (WES), Sanger sequencing not only saves on cost but also enhances efficiency when examining a single gene. Among the novel mutations reported in this study, one is a deletion and insertion mutation involving the coding and splicing region and the other two are nonsense mutations. The mutation c.333_360+14del42insCTT involved exon 2 and the splice donor site of second intron of the GRP143 gene, which may destroy the normal splicing and result in exon skipping or create apremature stop codon. In our study, the two nonsense mutations, p.W92X and p.R265X, would cause a truncated protein. Moreover, the truncated protein is likely to be degraded by nonsense-mediated mRNA decay (NMD), suggesting that both nonsense alleles are LOF. As only three patients were studied, the prevalence of GPR143 mutations in Chinese patients cannot be conclusively estimated. The GPR143 mutations reported to date in reported Chinese patients and our data are summarized (Table 2). We found that a deletion/insertion mutation involving the coding region was the most common mutation type (33.3%), followed by nonsense (23.8%) and splice site mutations (23.8%). Missense mutations only accounted for 19.0%. This mutation spectrum is quite different from what has been found in a western population, where approximately 48% of reported OA1 mutations were intragenic deletions, and 43% were missense and nonsense mutations. Bassi and colleagues found a diverse prevalence of large deletions between European (<10%) and North American (>50%) patients with OA116.
The OA1 patients in our study exhibited congenital nystagmus and poor visual acuity with hypopigmentation of the fundus. Iris hypopigmentation ranged from mild to normal. Severe foveal hypoplasia was the prominent clinical feature. The mosaic pattern of the carrier’s fundus is also useful for diagnosis, especially for young children who often are unable to cooperate with a fundus examination. It is unclear how the mutated GPR143 causes ocular abnormalities. The most prominent signs in affected Caucasians with OA1 are iris translucency, macular hypoplasia, and fundus hypopigmentation17,18,19,20. African-American males with OA1 have non-albinotic, moderately pigmented fundi and no translucency of the iris20. Japanese patients have fundus hypopigmentation at a level between that of Caucasian and African-American patients21,22. Therefore, it is postulated that the level of ocular hypopigmentation is related to ethnic origin. In Chinese, iris color is usually brown with little iris translucency, the clinical findings are similar to those in Japanese patients.
The ERG of proband F3-III:3 showed a scotopic b-wave close to the upper limit of normal which conformed to the phenotype. It has been reported that in albinos, amplitudes of the scotopic ERG responses may be greater than normal23, which has been postulated to result from the increased amount of stimulating light entering the eye through a hypopigmented anterior segment24. In Chinese patients, the pigmentation of the anterior segment is usually normal. We postulate that the relatively elevated scotopic b-wave results from increased scattering of light due to hypopigmentation of fundus. VEP tests have been used to reveal the abnormal optic decussation in albinos25. However, we didn’t observe a difference in the amplitudes of the P100 for all channels. Both proband F3-III:3 in this study and one OA1 patient in Fang’s study8 showed nonspecific VEP findings. Although, chiasmal misrouting is very common in oculocutaneous albinism, it is possible that the misrouting of chiasma is mild in Chinese OA1 patients or minor abnormal chiasmal decussation could not be recorded by VEP.
Iris and fundus hypopigmentation is mild in Chinese OA1 cases8. Most cases manifest poor vision at a very young age when it is difficult to perform a detailed clinical examination. OA1 could easily be misdiagnosed as other disease, such as LCA, achromatopsia or CMN. Liu et al.9 studied a large Chinese family with X-linked recessive inheritance. Without the classical phenotype of OA1, they found that nystagmus was the most prominent and only consistent finding. Patients in the family were initially misdiagnosed as CMN, a congenital abnormality that cause involuntary rhythmic oscillations of the eyes that occurs in the absence of any obvious disorders26. The patient from Family 2 was initially misdiagnosed as LCA, since LCA patients also present with poor vision and nystagmus, and clinical examinations are sometimes not available. Genetic testing has helped us establish the exact diagnosis finally. Therefore, the diagnosis of OA1 often requires comprehensive considerations, including retinal structure and function tests, and eventually molecular screening, especially when clinical findings are confusing18,27.
In summary, this report identified three novel GPR143 mutations in Chinese patients with OA1. We characterized the clinical manifestations of OA1 in these patients and confirmed that foveal hypoplasia played a vital role in the diagnosis of OA1. Color fundus photos and autofluorescence imaging for female carrier may provide important evidence for diagnosis. Genetic analysis of GPR143 and detailed clinical examinations are helpful in the early diagnosis and genetic counselling of OA1, avoiding unnecessary and inappropriate intervention.
Additional Information
How to cite this article: Zou, X. et al. Molecular genetic and clinical evaluation of three Chinese families with X-linked ocular albinism. Sci. Rep. 6, 33713; doi: 10.1038/srep33713 (2016).
References
Preising, M., Op de Laak, J. P. & Lorenz, B. Deletion in the OA1 gene in a family with congenital X linked nystagmus. Br J Ophthalmol. 85, 1098–1103 (2001).
Bassi, M. T. et al. Cloning of the gene for ocular albinism type 1 from the distal short arm of the X chromosome. Nat Genet. 10, 13–19 (1995).
Schiaffino, M. V. et al. The ocular albinism type 1 gene product is a membrane glycoprotein localized to melanosomes. Proc Natl Acad Sci USA 93, 9055–9060 (1996).
Palmisano, I. et al. The ocular albinism type 1 protein, an intracellular G protein-coupled receptor, regulates melanosome transport in pigment cells. Hum Mol Genet. 17, 3487–3501 (2008).
Schnur, R. E. et al. OA1 mutations and deletions in X-linked ocular albinism. Am J Hum Genet. 62, 800–809 (1998).
d’Addio, M. et al. Defective intracellular transport and processing of OA1 is a major cause of ocular albinism type 1. Hum Mol Genet. 9, 3011–3018 (2000).
Yan, N. et al. A novel nonsense mutation of the GPR143 gene identified in a Chinese pedigree with ocular albinism. PLoS One. 7, e43177 (2012).
Fang, S. et al. Novel GPR143 mutations and clinical characteristics in six Chinese families with X-linked ocular albinism. Mol Vis. 14, 1974–1982 (2008).
Liu, J. Y. et al. Identification of a novel GPR143 mutation in a large Chinese family with congenital nystagmus as the most prominent and consistent manifestation. J Hum Genet. 52, 565–570 (2007).
Cai, C. Y. et al. A novel splicing site mutation of the GPR143 gene in a Chinese X-linked ocular albinism pedigree. Genet Mol Res. 12, 5673–5679 (2013).
Xiao, X. & Zhang, Q. Iris hyperpigmentation in a Chinese family with ocular albinism and the GPR143 mutation. Am J Med Genet A. 149A, 1786–1788 (2009).
Wang, Y. et al. Identification of a novel mutation in a Chinese family with X-linked ocular albinism. Eur J Ophthalmol. 19, 124–128 (2009).
Pan, Q. et al. A novel mutation, c.494C>A (p.Ala165Asp), in the GPR143 gene causes a mild phenotype in a Chinese X-linked ocular albinism patient. Acta Ophthalmol (2015).
Han, R. et al. GPR143 Gene Mutations in Five Chinese Families with X-linked Congenital Nystagmus. Sci Rep. 5, 12031 (2015).
Hu, J., Liang, D., Xue, J., Liu, J. & Wu, L. A novel GPR143 splicing mutation in a Chinese family with X-linked congenital nystagmus. Mol Vis. 17, 715–722 (2011).
Bassi, M. T. et al. Diverse prevalence of large deletions within the OA1 gene in ocular albinism type 1 patients from Europe and North America. Hum Genet. 108, 51–54 (2001).
Kinnear, P. E., Jay, B. & Witkop, C. J. Jr. Albinism. Surv Ophthalmol. 30, 75–101 (1985).
Charles, S. J., Green, J. S., Grant, J. W., Yates, J. R. & Moore, A. T. Clinical features of affected males with X linked ocular albinism. Br J Ophthalmol. 77, 222–227 (1993).
Schnur, R. E. et al. Phenotypic variability in X-linked ocular albinism: relationship to linkage genotypes. Am J Hum Genet. 55, 484–496 (1994).
Oetting, W. S. New insights into ocular albinism type 1 (OA1): Mutations and polymorphisms of the OA1 gene. Hum Mutat. 19, 85–92 (2002).
Hayakawa, M., Kanai, A., Kato, K., Nakajima, A. & Takamori, K. A Japanese family of Nettleship Falls X-linked ocular albinism. Nippon Ganka Gakkai Zasshi. 94, 1181–1187 (1990).
Shiono, T., Tsunoda, M., Chida, Y., Nakazawa, M. & Tamai, M. X linked ocular albinism in Japanese patients. Br J Ophthalmol. 79, 139–143 (1995).
Krill, A. E. & Lee, G. B. The electroretinogram in albinos and carriers of the ocular albino trait. Arch Ophthalmol. 69, 32–38 (1963).
Wack, M. A., Peachey, N. S. & Fishman, G. A. Electroretinographic findings in human oculocutaneous albinism. Ophthalmology. 96, 1778–1785 (1989).
Hoffmann, M. B., Wolynski, B., Meltendorf, S., Behrens-Baumann, W. & Kasmann-Kellner, B. Multifocal visual evoked potentials reveal normal optic nerve projections in human carriers of oculocutaneous albinism type 1a. Invest Ophthalmol Vis Sci. 49, 2756–2764 (2008).
Kerrison, J. B., Vagefi, M. R., Barmada, M. M. & Maumenee, I. H. Congenital motor nystagmus linked to Xq26-q27. Am J Hum Genet. 64, 600–607 (1999).
Faugere, V., Tuffery-Giraud, S., Hamel, C. & Claustres, M. Identification of three novel OA1 gene mutations identified in three families misdiagnosed with congenital nystagmus and carrier status determination by real-time quantitative PCR assay. BMC Genet. 4, 1 (2003).
Acknowledgements
We gratefully acknowledge all participating patients and their family members. This work was supported by grants from the Foundation Fighting Blindness USA (CD-CL-0214-0631-PUMCH), the National Natural Science Foundation of China (81470669) and the Beijing Natural Science Foundation (7152116) to R.S.
Author information
Authors and Affiliations
Contributions
X.Z. wrote the main manuscript text and prepared Figures 1–4. R.S. collected the patients, designed and supported the study and revised the article. H.L. recorded the ERGs and VEPs for the patients. L.Y. and Z.S. captured OCT and FAF images of the patients. Z.Y. and H.L. performed the Sanger sequencing. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zou, X., Li, H., Yang, L. et al. Molecular genetic and clinical evaluation of three Chinese families with X-linked ocular albinism. Sci Rep 7, 33713 (2017). https://doi.org/10.1038/srep33713
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep33713
- Springer Nature Limited
This article is cited by
-
Novel mutations of the X-linked genes associated with early-onset high myopia in five Chinese families
BMC Medical Genomics (2023)
-
Identification of a novel GPR143 mutation in X-linked ocular albinism with marked intrafamilial phenotypic variability
Journal of Genetics (2018)