Abstract
Background
Pathogenic variants of G-protein coupled receptor 143 (GPR143) gene often leads to ocular albinism type I (OA1) characterized by nystagmus, iris and fundus hypopigmentation, and foveal hypoplasia. In this study, we identified a novel hemizygous nonsense mutation in GPR143 that caused an atypical manifestation of OA1.
Case presentation
We reported a large Chinese family in which all affected individuals are afflicted with poor visual acuity and foveal hypoplasia without signs of nystagmus. Fundus examination of patients showed an absent foveal reflex and mild hypopigmentation. The fourth grade of foveal hypoplasia and the reduced area of blocked fluorescence at foveal region was detected in OCT. OCTA imaging showed the absence of foveal avascular zone. In addition, the amplitude of multifocal ERG was reduced in the central ring. Gene sequencing results revealed a novel hemizygous mutation (c.939G > A) in GPR143 gene, which triggered p.W313X. However, no iris depigmentation and nystagmus were observed among both patients and carriers.
Conclusions
In this study, we reported a novel nonsense mutation of GPR143 in a large family with poor visual acuity and isolated foveal hypoplasia without nystagmus, which further expanded the genetic mutation spectrum of GPR143.
Similar content being viewed by others
Background
GPR143, also known as the ocular albinism type 1 (OA1) gene, encodes a 7TM G-protein coupled protein and is exclusively expressed by pigment cells. The mutation of GPR143 leaded to OA1, an X-linked type of albinism, which results in nystagmus, impaired visual acuity and foveal hypoplasia [1].
The form of albinism in OA1 patients affects the eye, especially iris and fundus, but the pigmentation of hair and skin is usually normal. The early sign of OA1 is mostly nystagmus that usually appears within 6 months after birth [2]. Since iris and fundus hypopigmentation is not obvious among Asians, OA1 is usually misdiagnosed as the congenital idiopathic nystagmus [3,4,5]. Up to date, more than one hundred mutations in the GPR143 gene have been identified in OA1 in the world, but GPR143 variants without nystagmus have been rarely reported [6].
In this study, we reported and characterized a large Chinese family, in which all the affected individuals are afflicted with poor visual acuity and foveal hypoplasia as the predominant manifestations, while no sign of nystagmus was detected. Gene sequencing results revealed the disease-causing gene to GPR143 with a novel point mutation (c.939G > A) in exon 8. Together, our results presented this previously unidentified mutation of GPR143 that caused the isolated foveal hypoplasia without nystagmus.
Case presentation
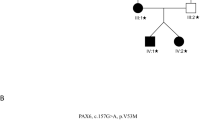
We identified a large, three-generation Chinese family with 25 living members, among whom 6 members were patients (Fig. 1A). All affected individuals were males, suggesting the inheritance pattern of the disease in the family was typical X-linked recessive.
The proband was misdiagnosed as amblyopia before he came to our hospital. The visual acuity of right and left eyes was 0.15 and 0.25, respectively, at his first visit. Fundus examination showed an absent foveal reflex and hypopigmentation in the entire retina (Fig. 1B). No iris depigmentation and nystagmus were observed.
OCT imaging showed grade 4 foveal hypoplasia. Grade 4 foveal hypoplasia is characterized by absence of foveal pit, absence of extrusion of plexiform layers and absence of lengthening of outer segments of photoreceptors [7]. OCT-angiography (OCTA) imaging showed the absence of foveal avascular zone (FAZ) (Fig. 2A). Fundus autofluorescence imaging (AFI) revealed the reduced area of blocked fluorescence at foveal region, indicating the macular pigment was severely affected (Fig. 1B). In addition, the amplitude of b-wave was attenuated in the full-field ERG recording, which is correlated with the dysfunction of photoreceptor respectively (Fig. 2B). VEP result was normal, without the typical pattern of VEP asymmetry showed in ocular albinism which correlates with the severity of albinism [8]. A poor fixation was revealed in microperimetry (The fixation stability within 4°: 33%), but the mean sensitivity was normal (Fig. 2C). In multifocal ERG, the amplitude was reduced in the central ring and flattened slope of amplitudes was observed from central to peripheral ring, indicating the depressed macular function (Fig. 2D).
Other affected individuals and obligate female carriers were also characterized clinically. Grade 4 foveal hypoplasia was identified in all patients, but not in carriers. Mild fundus hypopigmentation was observed in both patients and carriers. All family members were free from nystagmus and iris depigmentation (Table 1).
Furthermore, we screened for mutations on the genes from a specific hereditary eye disease enrichment panel based on targeted exome capture technology (Gencap) and next-generation sequencing (MyGenostics, Beijing). A hemizygous GPR143 variant (c.939G > A, nucleotide 939 in exon 8 is changed from guanine to adenine) was identified in the proband. This mutation belongs to the nonsense mutation (p.W313X). No cases of this mutation were retrieved in Human Gene Mutation Database. Sanger sequencing confirmed the mutation in all patients and showed a heterozygous mutation at this locus in the female carriers (Fig. 3A).
A glass prescription was given for full-time wear and the proband was instructed to return every year for follow up. Due to the irregular glass wear during 2018, corrected visual acuity was dropped at the third visit. After education, vision in the new glasses was improved and stable at around 0.5 in each eye at his last visit, suggesting the importance of the early-stage intervention of refractive errors to avoid amblyopic development (Table 2). Consistent with the result at the first visit, fundus examination showed no foveal landmark in either eye. Taken together, a diagnosis of isolated foveal hypoplasia was given.
Discussion
In this study, we identified a novel nonsense mutation c.939G > A (p.W313X) in exon 8 of GPR143 among patients with impaired visual acuity and foveal hypoplasia. More than 100 mutations of GPR143 have been detected in family and sporadic cases, which is commonly associated with OA1 characterized by nystagmus, ocular hypopigmentation and foveal hypoplasia. However, no symptom of nystagmus was observed in our cases, indicating variable severities may be attributed by different locations of mutation.
GPR143 encodes a pigment cell specific G protein-coupled receptor protein which is expressed mainly in melanosomes [9]. Sequencing analysis revealed the mutation of a G to A transition at nucleotide 939 of GPR143. The mutation leads to the substitution of tryptophan to a stop codon at codon 313 (p.W313X) which causes the loss of C-terminal. Subsequently, the motif (p.329–330) containing melanosomal membrane localization signal is destroyed, which dampens the function of intracellular melanosome biogenesis [10, 11]. Consistently, we observed the mild hypopigmentation in the fundus imaging of patients. In addition, the mild hypopigmentation was also found in the fundus of carriers, possibly due to X chromosome inactivation [12]. In GPR143-knockdown melanocyte (including RPE), the number and movement of melanosome were significantly decreased, while GPR143 overexpression stimulated the production of melanin content in a dose-dependent manner [13, 14]. This dosage-dependent effect of GPR143 on pigment biogenesis was also demonstrated in other human genetic studies that carrier females showed patches of hypopigmentation of the fundus but did not have symptoms of foveal hypoplasia and usually have good visual acuity [15,16,17].
The combination of AFI and fundus photography indicated the significant reduction of macular pigment in patients, while carriers were affected to a lesser extent. The physiological FAZ was also lost at the foveal region of the patient showed in OCTA imaging. It is well-established that both the formation of FAZ and the accumulation of macular pigment are the contributing factors of foveal development [18]. However, the association of these fovea-related factors to GPR143 remains largely elusive.
The expression of GPR143 is found in pigment cells, including retinal pigment epithelium. However, in the published single-cell transcriptome data of human fetal retina [19], we cannot find the restricted expression of GPR143 in any of the developing retinal subtypes (Fig. 3B). Therefore, the result suggested a potential paracrine effect of GPR143 signaling in RPE on the neighboring foveal development. In previous studies, L-DOPA was demonstrated as the endogenous ligand of GPR143 [20]. L-DOPA/GPR143 mediates the negatively control the vascularization by increasing the secretion of PEDF and reducing VEGF production [21]. The vascular inhibition of GPR143 signaling may subsequently lead to foveal formation. As for the effect on macular pigment, increasing evidence have been shown that GPR143 activity may protect against both onset and progression of age-related macular degeneration (AMD) [22, 23]. Low macular pigment is a key risk factor for AMD, which indicates the potential association between GPR143 signaling and macular pigment [24]. Therefore, it could be postulated that GPR143 signaling can extrinsically promote the foveal formation via attenuating retinal vascularization and increasing the production of macular pigment.
Conclusions
Foveal hypoplasia from GPR143 variants is usually associated with conditions, such as eye albinism and nystagmus. Isolated foveal hypoplasia has been rarely reported and the corresponding GPR143 mutation was unknown [6]. In our study, we have identified a novel mutation of GPR143 in Chinese family with nystagmus-free foveal hypoplasia, which further expanded the genetic mutation spectrum. Further investigation of the function of GPR143 in foveal development may help to elucidate the mechanisms of the disease and molecular targets for potential clinical treatment.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the SRA repository (https://www.ncbi.nlm.nih.gov/sra). The accession number is PRJNA707130.
Abbreviations
- GPR143:
-
G-protein coupled receptor 143
- OA1:
-
Ocular albinism type 1
- OS:
-
Outer segment
- OCT:
-
Optical coherence tomography
- OCTA:
-
Optical coherence tomography angiography
- FAZ:
-
Foveal avascular zone
- AFI:
-
Fundus autofluorescence imaging
- ERG:
-
Electroretinogram
- VEP:
-
Visual evoked potential
- RPE:
-
Retinal pigment epithelium
- PEDF:
-
Pigment epithelium-derived factor
- VEGF:
-
Vascular endothelial growth factor
- AMD:
-
Age-related macular degeneration
References
Bassi MT, Schiaffino MV, Renieri A, De Nigris F, Galli L, Bruttini M, et al. Cloning of the gene for ocular albinism type 1 from the distal short arm of the X chromosome. Nat Genet. 1995;10(1):13–9. https://doi.org/10.1038/ng0595-13.
Liu JY, Ren X, Yang X, Guo T, Yao Q, Li L, et al. Identification of a novel GPR143 mutation in a large Chinese family with congenital nystagmus as the most prominent and consistent manifestation. J Hum Genet. 2007;52(6):565–70. https://doi.org/10.1007/s10038-007-0152-3.
Zhou P, Wang Z, Zhang J, Hu L, Kong X. Identification of a novel GPR143 deletion in a Chinese family with X-linked congenital nystagmus. Mol Vis. 2008;14:1015–9.
Han R, Wang X, Wang D, Wang L, Yuan Z, Ying M, et al. GPR143 gene mutations in five Chinese families with X-linked congenital Nystagmus. Sci Rep. 2015;5(1):12031. https://doi.org/10.1038/srep12031.
Jia X, Yuan J, Jia X, Ling S, Li S, Guo X. GPR143 mutations in Chinese patients with ocular albinism type 1. Mol Med Rep. 2017;15(5):3069–75. https://doi.org/10.3892/mmr.2017.6366.
Giocanti-Auregan A, Witmer MT, Radcliffe NM, D'Amico DJ. Isolated foveal hypoplasia without nystagmus. Int Ophthalmol. 2014;34(4):877–80. https://doi.org/10.1007/s10792-014-9900-5.
Thomas MG, Kumar A, Mohammad S, Proudlock FA, Engle EC, Andrews C, et al. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography a predictor of visual acuity? Ophthalmology. 2011;118(8):1653–60. https://doi.org/10.1016/j.ophtha.2011.01.028.
Dorey SE, Neveu MM, Burton LC, Sloper JJ, Holder GE. The clinical features of albinism and their correlation with visual evoked potentials. Br J Ophthalmol. 2003;87(6):767–72. https://doi.org/10.1136/bjo.87.6.767.
Palmisano I, Bagnato P, Palmigiano A, Innamorati G, Rotondo G, Altimare D, et al. The ocular albinism type 1 protein, an intracellular G protein-coupled receptor, regulates melanosome transport in pigment cells. Hum Mol Genet. 2008;17(22):3487–501. https://doi.org/10.1093/hmg/ddn241.
Figueroa AG, McKay BS. A G-protein coupled receptor and macular degeneration. Cells. 2020;9(4):910. https://doi.org/10.3390/cells9040910.
McKay BS. Pigmentation and vision: is GPR143 in control? J Neurosci Res. 2019;97(1):77–87. https://doi.org/10.1002/jnr.24246.
Lee W, Schuerch K, Xie Y, Zernant J, Tsang SH, Sparrow JR, et al. Simultaneous expression of ABCA4 and GPR143 mutations: a complex phenotypic manifestation. Invest Ophthalmol Vis Sci. 2016;57(7):3409–15. https://doi.org/10.1167/iovs.16-19621.
Burgoyne T, O'Connor MN, Seabra MC, Cutler DF, Futter CE. Regulation of melanosome number, shape and movement in the zebrafish retinal pigment epithelium by OA1 and PMEL. J Cell Sci. 2015;128(7):1400–7. https://doi.org/10.1242/jcs.164400.
Chen T, Wang H, Liu Y, Zhao B, Zhao Y, Fan R, et al. Ocular albinism type 1 regulates Melanogenesis in mouse melanocytes. Int J Mol Sci. 2016;17(10):1596. https://doi.org/10.3390/ijms17101596.
Paavo M, Zhao J, Kim HJ, Lee W, Zernant J, Cai C, et al. Mutations in GPR143/OA1 and ABCA4 inform interpretations of short-wavelength and near-infrared fundus autofluorescence. Invest Ophthalmol Vis Sci. 2018;59(6):2459–69. https://doi.org/10.1167/iovs.18-24213.
Sepulveda-Vazquez HE, Villanueva-Mendoza C, Zenteno JC, Villegas-Ruiz V, Pelcastre-Luna E, Garcia-Aguirre G. Macular optical coherence tomography findings and GPR143 mutations in patients with ocular albinism. Int Ophthalmol. 2014;34(5):1075–81. https://doi.org/10.1007/s10792-014-9912-1.
Khan KN, Lord EC, Arno G, Islam F, Carss KJ, Raymond F, et al. Detailed retinal imaging in carriers of ocular albinism. Retina. 2018;38(3):620–8. https://doi.org/10.1097/IAE.0000000000001570.
Bringmann A, Syrbe S, Gorner K, Kacza J, Francke M, Wiedemann P, et al. The primate fovea: structure, function and development. Prog Retin Eye Res. 2018;66:49–84. https://doi.org/10.1016/j.preteyeres.2018.03.006.
Lu Y, Shiau F, Yi W, Lu S, Wu Q, Pearson JD, et al. Single-cell analysis of human retina identifies evolutionarily conserved and species-specific mechanisms controlling development. Dev Cell. 2020;53(4):473–91e479. https://doi.org/10.1016/j.devcel.2020.04.009.
Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008;6(9):e236. https://doi.org/10.1371/journal.pbio.0060236.
Falk T, Congrove NR, Zhang S, McCourt AD, Sherman SJ, McKay BS. PEDF and VEGF-A output from human retinal pigment epithelial cells grown on novel microcarriers. J Biomed Biotechnol. 2012;2012:278932.
Brilliant MH, Vaziri K, Connor TB Jr, Schwartz SG, Carroll JJ, McCarty CA, et al. Mining retrospective data for virtual prospective drug repurposing: L-DOPA and age-related macular degeneration. Am J Med. 2016;129(3):292–8. https://doi.org/10.1016/j.amjmed.2015.10.015.
Figueroa AG, Boyd BM, Christensen CA, Javid CG, McKay BS, Fagan TC, et al. Levodopa positively affects Neovascular age-related macular degeneration. Am J Med. 2021;134(1):122–8e123. https://doi.org/10.1016/j.amjmed.2020.05.038.
Ashraf M, Souka A, Adelman RA. Age-related macular degeneration: using morphological predictors to modify current treatment protocols. Acta Ophthalmol. 2018;96(2):120–33. https://doi.org/10.1111/aos.13565.
Acknowledgements
Not applicable.
Funding
This study was granted by National Natural Science Foundation of China (81870694, 61671242).
Author information
Authors and Affiliations
Contributions
M.C., W.F. performed patient recruitment and informed consent. X.M., S.Y. analyzed and interpreted sequencing data. M.C., Y.Y., Q.L., W.F. performed clinical data evaluation. X.M. wrote the manuscript. W.F. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University and strictly followed the Helsinki declaration. All participants provided written informed consent.
Consent for publication
Written informed consent was obtained from all participants (or the parents of their children under 16 years old). Written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mao, X., Chen, M., Yu, Y. et al. Identification of a novel GPR143 mutation in a large Chinese family with isolated foveal hypoplasia. BMC Ophthalmol 21, 156 (2021). https://doi.org/10.1186/s12886-021-01905-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-021-01905-7