Abstract
Polymorphisms in the methylenetetrahydrofolate reductase (MTHFR) play important roles in tumor development, progression and metastasis. Moreover, recent studies have reported that a number of 3′-UTR polymorphisms potentially bind to specific microRNAs in a variety of cancers. The aim of this study was to investigate the association of four MTHFR polymorphisms, 2572C>A [rs4846049], 4869C>G [rs1537514], 5488C>T [rs3737967] and 6685T>C [rs4846048] with colorectal cancer (CRC) in Koreans. A total of 850 participants (450 CRC patients and 400 controls) were enrolled in the study. The genotyping of MTHFR 3′-UTR polymorphisms was performed by polymerase chain reaction-restriction fragment length polymorphism analysis or TaqMan allelic discrimination assay. We found that MTHFR 2572C>A, 4869C>G and 5488C>T genotypes were substantially associated with CRC susceptibility. Of the potentially susceptible polymorphisms, MTHFR 2572C>A was associated with increased homocysteine and decreased folate levels in the plasma based on MTHFR 677CC. Our study provides the evidences for 3′-UTR variants in MTHFR gene as potential biomarkers for use in CRC prevention.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most common type of cancer and the second leading cause of cancer-related mortality in Western countries1. The prognosis of CRC patients is dependent on the tumor stage at the time of diagnosis. However, over 57% of patients have regional or distant spread of cancer cells at the time of diagnosis2. The pathogenesis of CRC usually follows a stepwise progression from benign adenoma to invasive adenocarcinoma3. In colorectal carcinogenesis, the unique molecular and genetic alterations that occur within cells result in a specific CRC phenotype4. This phenotype is connected to variable tumor behaviors that are relevant to the prognosis and the response to specific therapies4. As a result, the term “CRC” no longer refers to a single disease, but rather a heterogeneous group of diseases associated with a diversity of genetic/epigenetic backgrounds. In this respect, many ongoing studies are aimed at evaluating biomarkers as potential predictors of prognosis or response to therapy, which will most likely lead to the individualized management of the disease.
Folate (FA) is important for cell division and homeostasis due to its essential role in the synthesis of S-adenosyl-methionine, the methyl donor required for all methylation reactions in the cell. In addition to its function in cell homeostasis, FA has been hypothesized to play a role in carcinogenesis, especially in development of CRC5. Several mechanisms could underlie FA deficiency-mediated CRC, including DNA strand breaks, aberrant DNA methylation and impaired DNA repair. Thus, FA has been proposed as a possible candidate nutrient for CRC prevention6. Genetic variants in FA metabolism-related genes may modulate levels of this vitamin and influence risk of carcinogenesis. Furthermore, previous meta-analyses of numerous epidemiologic studies reported that FA was a determinant of CRC risk7.
The effect of several polymorphic genes involved in FA metabolism, including methylenetetrahydrofolate reductase (MTHFR) on CRC susceptibility and progression has been investigated8 MTHFR catalyzes the reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate; the latter is the methyl donor for the conversion of homocysteine (Hcy) to methionine, whereas the former and its derivatives, are essential cofactors for both thymidylate and de novo purine synthesis9,10. During de novo purine synthesis, thymidylate synthase with the FA binding site catalyzes the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP). This conversion is indispensable for the production of thymine, a nucleotide needed for DNA synthesis and repair11,12. Decreased MTHFR activity and expression lead to an accumulation of Hcy and/or deficiency of FA13.
There are two well-studied polymorphisms of the MTHFR gene, i.e., MTHFR 677C>T and 1298A>C. Despite the existence of a large body of data for studies of associations between these polymorphisms and CRC, findings of genetic associations have been inconsistent for a variety of conditions8. Recent studies have shown some clinical impacts of polymorphisms in the 3′-UTR of certain genes, which may potentially bind to specific microRNAs (miRNAs) in various cancers14,15,16,17. However, variants in the MTHFR 3′-UTR have not been extensively studied.
In the present study, four single nucleotide polymorphisms (SNPs) in the MTHFR 3′-UTR were identified by a database search; these are MTHFR 2572C>A (rs4846049), 4869C>G (rs1537514), 5488C>T (rs3737967) and 6685T>C (rs4846048). There were 17 MTHFR 3′-UTR SNPs with minor allele frequencies >5% in the global population. The 13 SNPs in the MTHFR 3′-UTR were excluded from this study due to following reasons: (1) lack of information for validation in the Asian population (rs35134728 and rs55780505), (2) below 5% of minor allele frequency in the Asian population (rs2184226, rs868014, rs2077360 and rs4845884) and (3) failure of genotyping conditions (rs1537516, rs1537515, rs2184227, rs3737966, rs3820192, rs11559040 and rs72640221). The minor allele frequencies of the four SNPs were all >5% in the Asian population. Little is known about their genetic associations with CRC. Therefore, we investigated whether these polymorphisms of the MTHFR 3′-UTR correlate with CRC susceptibility in Koreans.
Results
Genetic susceptibility of single and multiple markers
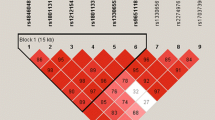
Table 1 presents demographic characteristics between cases and controls. Table 2 shows the distributions of genotypes and haplotypes for MTHFR 3′-UTR polymorphisms (2572C>A, 4869C>G, 5488C>T and 6685T>C) in CRC patients and control subjects. The genotype frequencies of controls and patients were consistent with expectations under Hardy-Weinberg equilibrium (HWE). Supplementary Table S1 shows the minor allele frequencies of MTHFR 3′-UTR polymorphisms described in previous reports. The allele frequencies of the control group in this study were similar to former results. MTHFR 2572CA + AA [adjusted odds ratio (AOR) = 1.49, 95% confidence interval (CI) = 1.10–2.03, P = 0.010], MTHFR 4869CG + GG (AOR = 2.17, 95% CI = 1.41–3.33, P < 0.001) and MTHFR 5488CT + TT (AOR = 1.66, 95% CI = 1.13–2.42, P = 0.001) were significantly associated with CRC susceptibility. The statistical significances remained after false discovery rate (FDR) correction by Benjamini-Hochberg method. Supplementary Table S2 shows AOR values of MTHFR 3′-UTR genotypes according to confounding variables. There were variations of AOR values for MTHFR 3′-UTR genotypes, but the statistical significances were unchanged for all conditions. To evaluate combined effects of MTHFR 3′-UTR SNP loci on CRC incidence, logistic regression for the combined genotypes and haplotypes was performed. The 2572CA + AA/4869CG + GG (AOR = 2.09, 95% CI = 1.35–3.24, P < 0.001), 4869CG + GG/5488CT + TT (AOR = 2.12, 95% CI = 1.38–3.27, P < 0.001) and 2572A-4869G-5488T-6685T (AOR = 1.99, 95% CI = 1.31–3.00, P = 0.001) types contributed to CRC prevalence (Table 3). However, the combined alleles for MTHFR 3′-UTR SNP loci did not show much stronger genetic associations than single genotypes.
Stratified effects of clinical and environmental factors
Unlike other cancers, CRC epidemiology was affected by a variety of identified risk factors in a complex manner3. Previous reports identified the following risk factors: aging, male gender, obesity, metabolic syndrome (MetS), hypertension (HTN), diabetes mellitus (DM), deficiency of FA, increased intake of red meat, excessive alcohol consumption and smoking3,18,19,20. In addition, epidemiologic alterations of CRC pathology and genetic differences correlating with the clinical features of CRC have been previously reported21,22,23. Therefore, stratified analyses were useful in elucidating CRC epidemiology resulting from a diversity of confounding variables. Except for FA deficiency, which presented difficulties in establishing a threshold using plasma levels, we conducted stratified analyses of the data according to age, gender, tumor site, tumor size, tumor node metastasis (TNM) stage, presence of MetS, HTN, DM, levels of body mass index (BMI), triglycerides (TG) and high density lipoprotein-cholesterol (HDL-C) to determine whether the 3′-UTR minor alleles were associated with CRC incidence in specific subsets of the study population. The results for the recessive model were excluded because of a small number of 3′-UTR minor homozygous genotypes when stratified by a variety of factors. FDR correction was used to eliminate false positive associations from stratified effects. Supplementary Table S3 summarizes the frequencies of MTHFR 3′-UTR genotypes in each stratified CRC group. The overall results of stratified analyses are shown in Table 4. MTHFR 2572CA + AA presented subset-specific associations in subgroups of patients with the following: ≥62 years of age (AOR = 1.72), male (AOR = 2.52), rectal cancer (AOR = 1.89), tumors ≥5 cm (AOR = 1.75), TNM stage I/II disease (AOR = 1.70), HTN (AOR = 2.03), DM (AOR = 2.03), and ≥150 mg/dL of TG (AOR = 2.20). MTHFR 4869CG + GG displayed subset-specific associations in subgroups of patients with the following: male (AOR = 5.05), rectal cancer (AOR = 2.70), tumors ≥5 cm (AOR = 2.64), TNM stage I/II disease (AOR = 2.33), HTN (AOR = 2.32), DM (AOR = 2.55), <25 kg/m2 of BMI (AOR = 2.12), ≥150 mg/dL of TG (AOR = 4.20), and ≥40 (male)/50 (female) mg/dL of HDL-C (AOR = 2.59). MTHFR 5488CT + TT showed subset-specific associations in subgroups of patients with the following: ≥62 years of age (AOR = 1.96), male (AOR = 2.99), rectal cancer (AOR = 2.02), tumors ≥5 cm (AOR = 2.02), TNM stage I/II disease (AOR = 1.84), HTN (AOR = 2.42), <25 kg/m2 of BMI (AOR = 2.12), and ≥40 (male)/50 (female) mg/dL of HDL-C (AOR = 1.86).
Combined effects of 3′-UTR polymorphisms with environmental factors
Because cancer risk is determined by the complex interplay of genetic and environmental factors, we calculated combined gene-environment effects on CRC susceptibility (Table 5 and Supplementary Tables S4–S7). To analyze combined gene-environment effects, we chose the following environmental risk factors which were significantly prevalent in the CRC group: lower plasma FA levels, MetS, HTN, DM and <40 (male)/50 (female) mg/dL of HDL-C. We used the relative excess odds due to interaction (RERIOR) to evaluate the additivity of odds between 3′-UTR genotypes and environmental risk factors24. With RERIOR >0, there was an additive interaction between the combined factors. All MTHFR 3′-UTR minor genotypes (2572CA + AA, 4869CG + GG, 5488CT + TT and 6685TC +CC) showed RERIOR >0 in combinations with <5.77 ng/mL of plasma FA (the lowest tertile interval), MetS and HTN (Table 5, Supplementary Tables S4 and S5). The 6685TC + CC genotype showed RERIOR <0 in combination with DM, whereas 4869CG + GG and 5488CT + TT genotypes showed RERIOR <0 in combination with lower HDL-C levels (Supplementary Tables S6 and S7). MTHFR 2572C>A was identified as the best polymorphism, showing additive interactions with the chosen environmental risk factors.
Variations of genetic associations for 2572C>A by 677C>T genotypes
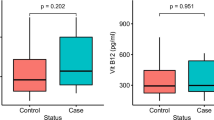
Next, we sought to determine whether the polymorphisms of interest within the 3′-UTR of the MTHFR gene correlated with plasma Hcy and FA concentrations (Table 6). We analyzed plasma Hcy and FA levels according to studied 3′-UTR polymorphisms based on MTHFR 677C>T, due to its strong associations with MTHFR activity13. The CC genotypes of MTHFR 677C>T and MTHFR 2572C>A showed significant correlations with increased plasma Hcy (regression coefficient = 1.120, t value = 3.361, P = 0.001) and decreased plasma FA (regression coefficient = −1.370, t = −2.595, P = 0.010) levels. MTHFR 6685T>C showed a correlation with decreased plasma FA (regression coefficient = −1.482, t = −2.428, P = 0.016) levels with the 677CC type. We also quantified expression of MTHFR mRNA in 47 tumor and tumor-adjacent tissue samples and searched for differences in expression based on the MTHFR genotype. The characteristics of 47 tumor and tumor-adjacent tissues are shown in Supplementary Table S8. The MTHFR 2572A allele had a tendency of decreased MTHFR mRNA expression [presenting lower –delta (Δ) cycle of threshold (CT) values derived from the equation as –ΔCT = –(CT MTHFR – CT 18S rRNA)] in tumor-adjacent tissues (Supplementary Table S9). Supplementary Table S10 shows MTHFR mRNA expression of 2572C>A based on the 677C>T genotypes. We excluded data for 4869C>G, 5488C>T and 6685T>C due to a small number of minor genotypes not reaching three individuals with stratified 677C>T genotypes. Similar to the results of Table 6, MTHFR 2572CA + AA displayed lower –ΔCT values of mRNA expression within the 677CC genotype. Because the functionality of MTHFR 2572C>A was affected by the 677C>T genotypes, we examined AOR values of 3′-UTR minor genotypes when stratified by 677C>T (Supplementary Table S11). MTHFR 2572CA + AA, 4869CG + GG and 5488CT + TT increased AOR values for CRC prevalence within the CT + TT genotype of 677C>T.
Discussion
In the present study, we investigated whether four 3′-UTR polymorphisms of the MTHFR gene are related to the occurrence of CRC. We found that MTHFR genotypes 2572C>A, 4869C>G and 5488C>T were substantially associated with CRC susceptibility and displayed significant combined gene-environment effects (RERIOR>0). Moreover, MTHFR 2572C>A was associated with increased Hcy and decreased FA levels in the plasma based on MTHFR 677CC genotype. To our knowledge, this is the study to provide evidence that 3′-UTR polymorphisms of MTHFR gene are associated with CRC susceptibility.
Numerous studies have investigated the associations of MTHFR genetic polymorphisms with CRC incidence8,25. Many reports have demonstrated inconsistent data for the significance of MTHFR 677C>T, although the MTHFR 677T allele was reported to be a potential genetic risk factor for increased CRC susceptibility, as determined in a recent meta-analysis25. There were limited conditions for the association of the MTHFR 677T allele with increased CRC risk: (1) high alcohol intake26,27, (2) low FA intake26,28 and (3) microsatellite instability (MSI)29,30,31. Although there was no similarity when the present study design was compared with numerous previous reports, the subset-specific associations of 2572C>A, 4869C>G and 5488C>T polymorphisms were observed. We found common subset-specific associations in the subgroup of patients with the following: ≥62 years of age, male, rectal cancer, tumors ≥5 cm, TNM stage I/II disease and HTN. We hypothesized the reasons for these correlations were high alcohol intake, low FA intake and microsatellite instability. Korean men showed higher rates of alcohol consumption than women32, FA deficiency increased with age33 and FA intake showed stronger associations with decreased risk for the cancer of the rectum than of the colon34. HTN may be prevented by FA fortification35 and increased prevalence of tumors ≥5 cm were associated with MSI36. Finally, CRC with early TNM stage and without lymph node metastases showed a higher frequency of MSI37.
The MTHFR 677T allele decreased MTHFR activity correlated with increased plasma Hcy and decreased plasma FA levels13. Also, we observed that MTHFR 2572A allele had a tendency of decreased MTHFR mRNA expression in tumor-adjacent tissues. Therefore, it is necessary to gain an understanding of carcinogenic events caused by decreased MTHFR activity to explain why 3′-UTR polymorphisms may affect CRC susceptibility. Lower MTHFR activity increases Hcy and decrease FA levels in the plasma, inducing development of CRC38,39. Plasma FA concentration inversely correlates with Hcy level40. Depletion of FA may be considered a risk factor for colorectal carcinogenesis because it induces breaks in human chromosomal DNA41. Two plausible mechanisms by which FA deficiency may create such breaks are uracil misincorporation and impaired DNA repair42,43. FA deficiency reduces synthesis of deoxythymidylate from deoxyuridylate and the resultant nucleotide imbalance accelerates the incorporation of uracil into DNA. Uracil in DNA is excised by a repair glycosylase and, in the process, a transient single-strand break develops in the DNA44. Simultaneous removal and repair of two adjacent uracil residues on opposite strands can cause a double-strand break, which is difficult to repair and further increases genetic instability. Unrepaired double-strand DNA breaks enhance cellular transformation and increase cancer risk44. FA status is also important to modify cell proliferation rates. James et al. reported excessive cell proliferation in livers of FA/methyl-deficient rats45. Conversely, FA supplementation has been found to diminish colorectal mucosal proliferation in both animal and human studies. Nensey et al. reported the same phenomenon in an animal model46. FA supplementation reduces carcinogen-induced ornithine decarboxylase and tyrosine kinase activities, both of which are indicators of cell proliferation46. Biasco et al.47 reported that FA supplementation significantly decreases rectal mucosal proliferation in patients with long-standing ulcerative colitis, a condition that carries a higher risk of CRC, a predisposition to which is considered to be due in part to reduced availability of FA. Akoglu et al.48 described a human colon cancer cell line in which dihydrofolate and methyl-THF serve as growth-inhibitory factors.
Epidemiologic studies over the past two or three decades have described an inverse relationship between FA status (assessed by dietary FA intake or measurement of red cell and plasma FA levels) with the risk of cancer of the lungs, oropharynx, esophagus, stomach, colorectum, pancreas, cervix, ovary, prostate and breast and the risk of neuroblastoma and leukemia49,50. Although the results of epidemiologic and clinical studies are inconsistent, the reports have indicated 20%–40% lower risk of CRC in subjects with the highest dietary intake or blood levels of folate compared with those with the lowest intake or blood levels49,51,52. Several intervention studies have shown that FA supplementation can improve or reverse poor prognostic factors of CRC49,51 and some epidemiologic studies have shown a beneficial effect of taking multivitamin supplements containing ≥400 μg FA on CRC risk and mortality53,54,55. The data from animal studies generally support a causal association between FA deficiency and CRC risk and an inhibitory effect of modest levels of FA supplementation on colorectal carcinogenesis51. The results for previous association studies of plasma Hcy or FA levels with risk of colorectal neoplasia are complicated. Four studies reported increased risk of colorectal neoplasia by higher plasma Hcy or lower plasma FA levels56,57,58,59, Kato et al.56 and Pufulete et al.57 demonstrated associations with decreased plasma FA levels whereas Ulvik et al.58 and Martinez et al.59 presented significant relationships with higher plasma Hcy levels. Moreover, Martinez et al. reported that the risk of colorectal neoplasia could be controlled by FA supplementation59. Three other studies did not report a significantly elevated risk of colorectal neoplasia with increasing plasma Hcy or decreasing plasma FA levels60,61,62. In this study, there were the additive interactions of CRC risk (RERIOR >0) between MTHFR 3′-UTR minor genotypes and lower plasma FA levels (<5.77 ng/mL: the lowest tertile interval). FA supplementation or fortification may therefore be essential for the prevention of colorectal carcinogenesis in individuals with MTHFR 3′-UTR minor genotypes.
Genetic variation in the 3′-UTR region could affect the stability and translation of the mRNA through altered miRNA-binding affinity. In the present study, we could not demonstrate altered miRNA-binding activity depending on MTHFR 3′-UTR polymorphisms. One study showed altered miR-149–binding activity with polymorphism MTHFR 2572C>A63. The MTHFR 2572A allele augmented miR-149–binding activity associated with decreased MTHFR expression. Further studies are needed to directly test for binding activity of miRNA to MTHFR 3′-UTR polymorphic regions to determine the mechanism by which these polymorphisms may influence cellular proliferation and cancer progression. These studies may have great clinical impact for all diseases related to one-carbon metabolism.
In conclusion, we investigated the relationship of the polymorphisms MTHFR 2572C>A, 4869C>G, 5488C>T and 6685T>C with CRC susceptibility. We found specific 3′-UTR polymorphisms positively correlated with CRC susceptibility, depending on a diversity of clinical and environmental risk factors. This study has several limitations. First, the manner in which 3′-UTR polymorphisms in the MTHFR gene affect development of CRC is still unclear. Second, information regarding additional environmental risk factors in CRC patients is lacking and remains to be investigated. Third, a limited number of 3′-UTR polymorphisms were studied. Lastly, the population of this study was limited to ethnic Koreans. Although results of our study provide the first evidence for 3′-UTR variants in the MTHFR gene as potential biomarkers for use in CRC prevention, a prospective study on a larger cohort of ethnically diverse patients is warranted to validate these findings.
Methods
Ethics statement
The study protocol was approved by the Institutional Review Board of CHA Bundang Medical Center. All study subjects provided written informed consent to participate in the study. All the methods applied in the study were carried out in accordance with the approved guidelines.
Study population
A case–control study of 850 individuals was conducted. Four hundred and fifty patients diagnosed with CRC at CHA Bundang Medical Center, Seongnam, South Korea were enrolled from June 2005 to January 2010. This study only included CRC patients who had undergone surgical resection with a curative intent and who had histologically confirmed adenocarcinoma. The response rate of CRC patients, with an initial number of 598 individuals, who gave written informed consent was 75.3%. Within the CRC cohort, 155 consecutive patients with proximal colon cancer (i.e., from the cecum to the splenic flexure), 101 consecutive patients with distal colon cancer (i.e., descending and sigmoid colon), 8 consecutive patients with mixed colon cancer and 186 consecutive patients with rectal cancer underwent primary surgery. Information concerning the date of diagnosis and pathological stage was obtained retrospectively. Tumor staging was performed according to the sixth edition of the American Joint Committee on Cancer (AJCC) staging manual. The control group consisted of 400 individuals randomly selected following a health screening. Of the initial 612 normal visitors, 476 individuals gave written informed consent (response rate: 77.8%) and 76 participants were excluded from the study due to a history of thrombotic diseases or cancer. Patients with a high baseline blood pressure (systolic ≥140 mm Hg or diastolic ≥90 mm Hg) on more than one occasion or a history of treatment with antihypertensive medication were classified as having HTN. Patients with high fasting plasma glucose ( ≥126 mg/dl) and those who took oral hypoglycemic agents or who had a history of insulin treatment were classified as having DM. Individuals were diagnosed with MetS if they possessed three or more of the following five risk factors: BMI ≥25.0 kg/m2; TG ≥150 mg/dl; HDL-C <40 mg/dl for men or <50 mg/dl for women; blood pressure ≥130/85 mm Hg or currently taking anti-hypertension medication; and fasting blood sugar ≥100 mg/dl or currently taking hypoglycemic medication.
Phenotype measurements
Anthropometric measurements included BMI. Systolic and diastolic blood pressures of subjects were measured in the seated position after 10 min of rest. For measurements of physiological parameters, 3-ml samples of blood were obtained after fasting overnight. Plasma glucose was determined in duplicate using the hexokinase method adapted for an automated analyzer (TBA 200FR NEO, Toshiba Medical Systems, Tokyo, Japan). Levels of TG and HDL-C were determined by enzymatic colorimetric methods using commercial reagent sets (Toshiba Medical Systems). The concentration of Hcy in the plasma was measured by fluorescence polarization immunoassay (FPIA) with the IMx automated analyzer (Abbott Laboratories, Chicago, IL, USA). The plasma concentration of FA was determined using a radioassay kit (ACS:180, Bayer, Tarrytown, NY, USA).
Genotyping
DNA was extracted from leukocytes using a G-DEX™ II Genomic DNA Extraction kit (Intron Biotechnology, Seongnam, Korea) according to the manufacturer’s instructions. Genotyping of the MTHFR 677C>T, MTHFR 5488C>T and MTHFR 6685T>C was carried out using real-time polymerase chain reaction (PCR) (RG-6000, Corbett Research, Australia) for allelic discrimination, Genotyping of MTHFR 2572C>A and MTHFR 4869C>G was carried out by PCR-restriction fragment length polymorphism (PCR-RFLP) analysis. Primers and TaqMan probes were designed using Primer Express Software (version 2.0) and were synthesized and supplied by Applied Biosystems (Carlsbad, CA, USA). The reporter dyes used were FAM and JOE. Primers for amplification of MTHFR 677C>T were (forward) 5′- TGA CCT GAA GCA CTT GAA GGA GAA -3′ and (reverse) 5′- GGA AGA ATG TGT CAG CCT CAA AGA -3′. Selected probes were 5′-FAM-ATG AAA TCG GCT CCC G -TAMRA-3′ (C-allele–detecting) and 5′-JOE-ATG AAA TCG ACT CCC G -TAMRA-3′ (T-allele–detecting). Primers for amplification of MTHFR 5488C>T were (forward) 5′- GAG GCA CCA GCT CTG TGG -3′ and (reverse) 5′- CCC CAG GAA GTC CAA GC -3′. The selected probes were 5′-FAM- CAG CAG CTG CGG GTC TGA A -TAMRA-3′ (C-allele–detecting) and 5′-JOE- CAG CAG CTG TGG GTC TGA A -TAMRA-3′ (T-allele–detecting). Primers for amplification of MTHFR 6685T>C were (forward) 5′- CCA GAC CAG AAG CAG TTA -3′ and (reverse) 5′- GCT GTG CAG TGT CAT TT -3′. The selected probes were 5′-FAM- CAC CAA CAA ATG GTG ATA AG -TAMRA-3′ (T-allele–detecting) and 5′-JOE- CAC CAA CAA GTG GTG ATA AG -TAMRA-3′ (C-allele–detecting). MTHFR 2572C>A was amplified using forward (5′- TTG CCA ACT AAG CCC TCG AAA CAA -3′) and reverse (5′- TGC CAC ATC TCT TCT ACG ATG CCA -3′) primers. The 140-bp PCR product was then digested with 5U StyI. A digestion product of 140 bp represented the AA genotype; fragments of 140 and 70 bp represented the CA genotype; and 70-bp products represented the CC genotype. MTHFR 4869C>G was amplified using forward (5′- AGG CAA GCC CCT CAG CCC TT -3′) and reverse (5′- TCC AGC CCT GAG CCC AGA GTC T -3′) primers. The 126-bp product was digested with 3U BsmAI for 16 h at 55 °C. A restriction fragment of 126 bp represented the CC genotype; fragments of 126 bp, 98 bp and 28 bp represented the CG genotype; and 98-bp and 28-bp products represented the GG genotype. All genotyping data of study participants were confirmed three times to eliminate genotyping errors. Thirty percent of the PCR assays for each polymorphism were randomly selected and repeated, followed by DNA sequencing, to validate the experimental findings. Sequencing was performed using an ABI 3730xl DNA Analyzer (Applied Biosystems, Carlsbad, CA, USA). The concordance of the quality control samples was 100%.
Quantitative real-time PCR
To perform quantitative real-time PCR (qRT-PCR), total RNA was extracted from 47 tumor and tumor-adjacent tissues of CRC patients using TRIzol Reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s instructions. cDNA was made from total RNA using the SuperScript III First-Strand Synthesis System (Invitrogen). Quantitation of MTHFR mRNA was determined using real-time PCR (RG-6000, Corbett Research, Australia). The MTHFR mRNA expression ratio (amount of MTHFR mRNA/amount of 18S rRNA) was expressed as –ΔCT = –(CT MTHFR – CT 18S rRNA). Housekeeping 18S rRNA was used as an internal control. The primer sequences for amplification were as follows: 18S rRNA, (forward) 5′- AAC TTT CGA TGG TAG TCG CCG -3′ and (reverse) 5′- CCT TGG ATG TGG TAG CCG TTT -3′; MTHFR, (forward) 5′- GAA CGA AGC CAG AGG AAA CA -3′ and (reverse) 5′- GGG TGG AAC ATC TCG AAC TAT C -3′.
Statistical analysis
To analyze baseline characteristics, chi-square tests were used for categorical data and Student’s t-tests were used for continuous data to compare patient and control baseline data. Association of MTHFR 3′-UTR polymorphisms with CRC incidence was calculated using adjusted odds ratios (AORs) and 95% confidence intervals (CIs) from multivariate logistic regression adjusted for age, gender, HTN, DM, BMI, TG and HDL-C. These parameters were selected as adjustment variables because they were directly or potentially associated with CRC3,18,19,20. To evaluate the association data by the Benjamini-Hochberg method, we calculated FDR-corrected P values according to the number of genetic markers and stratified groups64. RERIOR was used to calculate additive interactions between genotypes and environmental risk factors24. When RERIOR >0, there was an additive interaction between combined factors. Correlation of MTHFR 3′-UTR polymorphisms with plasma Hcy and FA levels was calculated using regression coefficients and t values from multivariate linear regression adjusted for age, gender, HTN, DM, BMI, TG and HDL-C. The statistical significances of MTHFR mRNA expression levels according to studied polymorphisms and tissue differences were calculated by Mann-Whitney, Kruskal-Wallis and Wilcoxon tests. The haplotypes for multiple loci were estimated using the expectation maximization algorithm with SNPAlyze (version 5.1; DYNACOM Co, Ltd, Yokohama, Japan) and those with frequencies of <1% were excluded from statistical analysis. Analyses were performed using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA) and Medcalc version 12.7.1.0 (Medcalc Software, Mariakerke, Belgium).
Additional Information
How to cite this article: Jeon, Y. J. et al. Genetic variants in 3'-UTRs of methylenetetrahydrofolate reductase (MTHFR) predict colorectal cancer susceptibility in Koreans. Sci. Rep. 5, 11006; doi: 10.1038/srep11006 (2015).
References
Jemal, A. et al. Cancer statistics, 2009. CA Cancer J Clin 59, 225–249 (2009).
Figueredo, A., Coombes, M. E. & Mukherjee, S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev 2008, CD005390 (2008).
Brenner, H., Kloor, M. & Pox, C. P. Colorectal cancer. Lancet 383, 1490–1502 (2014).
Zoratto, F. et al. Focus on genetic and epigenetic events of colorectal cancer pathogenesis: implications for molecular diagnosis. Tumour Biol 35, 6195–6206 (2014).
Duthie, S. J. et al. Folate, DNA stability and colo-rectal neoplasia. Proc Nutr Soc 63, 571–578 (2004).
Choi, S. W. & Mason, J. B. Folate status: effects on pathways of colorectal carcinogenesis. J Nutr 132, 2413S–24188S (2002).
Kennedy, D. A., et al. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer Epidemiol 35, 2–10 (2011).
Figueiredo, J. C., Levine, A. J., Crott, J. W., Baurley, J. & Haile, R. W. Folate-genetics and colorectal neoplasia: what we know and need to know next. Mol Nutr Food Res 57, 607–627 (2013).
Slattery, M. L., Potter, J. D., Samowitz, W., Schaffer, D. & Leppert, M. Methylenetetrahydrofolate reductase, diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev 8, 513–518 (1999).
Kono, S. & Chen, K. Genetic polymorphisms of methylenetetrahydrofolate reductase and colorectal cancer and adenoma. Cancer Sci 96, 535–542 (2005).
Kawakami, K., Omura, K., Kanehira, E. & Watanabe, Y. Polymorphic tandem repeats in the thymidylate synthase gene is associated with its protein expression in human gastrointestinal cancers. Anticancer Res 19, 3249–3252 (1999).
Mandola, M. V. et al. A 6 bp polymorphism in the thymidylate synthase gene causes message instability and is associated with decreased intratumoral TS mRNA levels. Pharmacogenetics 14, 319–327 (2004).
van der Put, N. M. et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural–tube defects? Am J Hum Genet 62, 1044–1051 (1998).
Wang, L. et al. A miRNA binding site single-nucleotide polymorphism in the 3′-UTR region of the IL23R gene is associated with breast cancer. PLoS One 7, e49823 (2012).
Sebio, A. et al. The LCS6 polymorphism in the binding site of let-7 microRNA to the KRAS 3′-untranslated region: its role in the efficacy of anti-EGFR-based therapy in metastatic colorectal cancer patients. Pharmacogenet Genomics 23, 142–147 (2013).
Yang, P. et al. A functional variant at miR-24 binding site in B7-H2 alters susceptibility to gastric cancer in a Chinese Han population. Mol Immunol 56, 98–103 (2013).
Zhou, L. et al. Association of a genetic variation in a miR-191 binding site in MDM4 with risk of esophageal squamous cell carcinoma. PLoS One 8, e64331 (2013).
Giovannucci, E. Metabolic syndrome, hyperinsulinemia and colon cancer: a review. Am J Clin Nutr 86, s836–842 (2007).
Baena, R. & Salinas, P. Diet and colorectal cancer. Maturitas 80, 258–264 (2015).
Shin, A. et al. Risk prediction model for colorectal cancer: National Health Insurance Corporation study, Korea. PLoS One 9, e88079 (2014).
Singh, S., Singh, P. P., Murad, M. H., Singh, H. & Samadder, N. J. Prevalence, risk factors and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol 109, 1375–1389 (2014).
Ju, J. H. et al. Changes in disease pattern and treatment outcome of colorectal cancer: a review of 5,474 cases in 20 years. Int J Colorectal Dis 22, 855–862 (2007).
Mates, I. N. et al. Single nucleotide polymorphisms in colorectal cancer: associations with tumor site and TNM stage. J Gastrointestin Liver Dis 21, 45–52 (2012).
Chu, H., Nie, L. & Cole, S. R. Estimating the relative excess risk due to interaction: a bayesian approach. Epidemiology 22, 242–248 (2011).
Teng, Z. et al. The 677C>T (rs1801133) polymorphism in the MTHFR gene contributes to colorectal cancer risk: a meta-analysis based on 71 research studies. PLoS One 8, e55332 (2013).
Levine, A. J. et al. The methylenetetrahydrofolate reductase 677C-->T polymorphism and distal colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev 9, 657–663 (2000).
Giovannucci, E. et al. Methylenetetrahydrofolate reductase, alcohol dehydrogenase, diet and risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev 12, 970–979 (2003).
Marugame, T. et al. Relation of plasma folate and methylenetetrahydrofolate reductase C677T polymorphism to colorectal adenomas. Int J Epidemiol 32, 64–66 (2003).
Levine, A. J. et al. Genetic variability in the MTHFR gene and colorectal cancer risk using the colorectal cancer family registry. Cancer Epidemiol Biomarkers Prev 19, 89–100 (2010).
Naghibalhossaini, F. et al. MTHFR C677T and A1298C variant genotypes and the risk of microsatellite instability among Iranian colorectal cancer patients. Cancer Genet Cytogenet 197, 142–151 (2010).
Hubner, R. A., Lubbe, S., Chandler, I. & Houlston, R. S. MTHFR C677T has differential influence on risk of MSI and MSS colorectal cancer. Hum Mol Genet 16, 1072–1077 (2007).
Ryu, S. Y., Crespi, C. M. & Maxwell, A. E. Drinking patterns among Korean adults: results of the 2009 Korean community health survey. J Prev Med Public Health 46, 183–191 (2013).
Mézière, A. et al. B12 deficiency increases with age in hospitalized patients: a study on 14,904 samples. J Gerontol A Biol Sci Med Sci 69, 1576–1585 (2014).
Brink, M. et al. Dietary folate intake and k-ras mutations in sporadic colon and rectal cancer in The Netherlands Cohort Study. Int J Cancer 114, 824–830 (2005).
Xun, P. et al. Folate intake and incidence of hypertension among American young adults: a 20-y follow-up study. Am J Clin Nutr 95, 1023–1030 (2012).
Søreide, K. et al. Microsatellite instability and DNA ploidy in colorectal cancer: potential implications for patients undergoing systematic surveillance after resection. Cancer 115, 271–282 (2009).
Kazama, Y. et al. Microsatellite instability in poorly differentiated adenocarcinomas of the colon and rectum: relationship to clinicopathological features. J Clin Pathol 60, 701–704 (2007).
Chen, F. P., Lin, C. C., Chen, T. H., Tsai, M. C. & Huang, Y. C. Higher plasma homocysteine is associated with increased risk of developing colorectal polyps. Nutr Cancer 65, 195–201 (2013).
Miller, J. W. et al. Homocysteine, cysteine and risk of incident colorectal cancer in the Women’s Health Initiative observational cohort. Am J Clin Nutr 97, 827–834 (2013).
Brevik, A. et al. Plasma concentration of folate as a biomarker for the intake of fruit and vegetables: the Hordaland Homocysteine Study. Am J Clin Nutr 81, 434–439 (2005).
Branda, R. F. & Blickensderfer, D. B. Folate deficiency increases genetic damage caused by alkylating agents and gamma–irradiation in Chinese hamster ovary cells. Cancer Res 53, 5401–5408 (1993).
Duthie, S. J., Narayanan, S., Blum, S., Pirie, L. & Brand, G. M. Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells. Nutr Cancer 37, 245–251 (2000).
Choi, S. W., Kim, Y. I., Weitzel, J. N. & Mason, J. B. Folate depletion impairs DNA excision repair in the colon of the rat. Gut 43, 93–99 (1998).
Blount, B. C. et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci USA 94, 3290–3295 (1997).
James, S. J. et al. Apoptosis and proliferation under conditions of deoxynucleotide pool imbalance in liver of folate/methyl deficient rats. Carcinogenesis 18, 287–293 (1997).
Nensey, Y. M., Arlow, F. L. & Majumdar, A. P. Aging. Increased responsiveness of colorectal mucosa to carcinogen stimulation and protective role of folic acid. Dig Dis Sci 40, 396–401 (1995).
Biasco, G. et al. Folic acid supplementation and cell kinetics of rectal mucosa in patients with ulcerative colitis. Cancer Epidemiol Biomarkers Prev 6, 469–471 (1997).
Akoglu, B., Faust, D., Milovic, V. & Stein, J. Folate and chemoprevention of colorectal cancer: Is 5-methyl-tetrahydrofolate an active antiproliferative agent in folate-treated colon-cancer cells? Nutrition 17, 652–653 (2001).
Kim, Y. I. Folate and carcinogenesis: evidence, mechanisms and implications. J Nutr Biochem 10, 66–88 (1999).
Kim, Y. I. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr 80, 1123–1128 (2004).
Kim, Y. I. Role of folate in colon cancer development and progression. J Nutr 133, 3731S–3739S (2003).
Giovannucci, E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr 132, 2350S–2355S (2002).
Giovannucci, E. et al. Alcohol, low-methionine–low-folate diets and risk of colon cancer in men. J Natl Cancer Inst 87, 265–273 (1995).
Giovannucci, E. et al. Multivitamin use, folate and colon cancer in women in the Nurses’ Health Study. Ann Intern Med 129, 517–524 (1998).
Jacobs, E. J. et al. Multivitamin use and colon cancer mortality in the Cancer Prevention Study II cohort (United States). Cancer Causes Control 12, 927–934 (2001).
Kato, I. et al. Serum folate, homocysteine and colorectal cancer risk in women: a nested case-control study. Br J Cancer 79, 1917–1922 (1999).
Pufulete, M. et al. Folate status, genomic DNA hypomethylation and risk of colorectal adenoma and cancer: a case control study. Gastroenterology 124, 1240–1248 (2003).
Ulvik, A. et al. Colorectal cancer and the methylenetetrahydrofolate reductase 677C –>T and methionine synthase 2756A –>G polymorphisms: a study of 2,168 case-control pairs from the JANUS cohort. Cancer Epidemiol Biomarkers Prev 13, 2175–2180 (2004).
Martínez, M. E. et al. Folate fortification, plasma folate, homocysteine and colorectal adenoma recurrence. Int J Cancer 119, 1440–1446 (2006).
Van Guelpen, B. et al. Low folate levels may protect against colorectal cancer. Gut 55, 1461–1466 (2006).
Weinstein, S. J. et al. One-carbon metabolism biomarkers and risk of colon and rectal cancers. Cancer Epidemiol Biomarkers Prev 17, 3233–3240 (2008).
Le Marchand, L. et al. Plasma levels of B vitamins and colorectal cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 18, 2195–2201 (2009).
Wu, C. et al. The human MTHFR rs4846049 polymorphism increases coronary heart disease risk through modifying miRNA binding. Nutr Metab Cardiovasc Dis 23, 693–698 (2013).
Benjamini, Y., Drai, D., Elmer, G., Kafkafi, N. & Golani, I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125, 279–284 (2001).
Acknowledgements
This study was supported by National Research Foundation of Korea (NRF) funded by the Ministry of Education (2009-0093821 and 2012R1A1A2007033).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: J.Y.J., K.J.W. and K.N.K. Performed the experiments: J.Y.J., P.H.M., J.H.G. and K.J.O. Analyzed the data and statistical analyses: J.Y.J., K.J.W., K.N.K., P.H.M. and K.J.O. Contributed reagents/material/analysis tools: K.J.W., O.J., H.S.G., K.S.W., O.D. and K.N.K. Wrote the main manuscript text: J.Y.J., K.J.W. and K.N.K. Reference collection and data management: K.J.W., O.J., H.S.G., K.S.W., O.D. and K.N.K. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jeon, Y., Kim, J., Park, H. et al. Genetic variants in 3′-UTRs of methylenetetrahydrofolate reductase (MTHFR) predict colorectal cancer susceptibility in Koreans. Sci Rep 5, 11006 (2015). https://doi.org/10.1038/srep11006
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep11006
- Springer Nature Limited