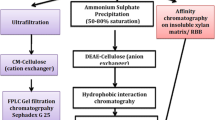
Xylose isomerase produced by Bacillus thermoantarcticus was purified 73-fold to homogeneity and its biochemical properties were determined. It was a homotetramer with a native molecular mass of 200 kDa and a subunit molecular mass of 47 kDa, with an isoelectric point at 4.8. The enzyme had a K m of 33 mM for xylose and also accepted D-glucose as substrate. Arrhenius plots of the enzyme activity of xylose isomerase were linear up to a temperature of 85°C. Its optimum pH was around 7.0, and it had 80% of its maximum activity at pH 6.0. This enzyme required divalent cations for its activity and thermal stability. Mn2+, Co2+ or Mg2+ were of comparable efficiency for xylose isomerase reaction, while Mg2+ was necessary for glucose isomerase reaction. Journal of Industrial Microbiology & Biotechnology (2001) 27, 234–240.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 18 March 2001/ Accepted in revised form 03 July 2001
Rights and permissions
About this article
Cite this article
Lama, L., Nicolaus, B., Calandrelli, V. et al. Purification and characterization of thermostable xylose(glucose) isomerase from Bacillus thermoantarcticus . J Ind Microbiol Biotech 27, 234–240 (2001). https://doi.org/10.1038/sj.jim.7000182
Issue Date:
DOI: https://doi.org/10.1038/sj.jim.7000182