Abstract
Filamentous fungi produce polysaccharide-degrading enzymes, which is controlled by poorly understood transcriptional circuits. Here we show that a circuit comprising RsrC-RsrA-RsrB (Rsr: production of raw-starch-degrading enzyme regulator) that positively regulates production of raw starch-degrading enzymes in Penicillium oxalicum. Transcription factor (TF) RsrA is essential for biosynthesis of raw starch-degrading enzymes. RsrB and RsrC containing Zn2Cys6- and C2H2-zinc finger domains, act downstream and upstream of RsrA, respectively. RsrA activates rsrB transcription, and three nucleotides (G-286, G-287 and G-292) of rsrB promoter region are required for RsrA, in terms of TF, for binding. RsrB165−271 binds to DNA sequence 5’-TCGATCAGGCACGCC-3’ in the promoter region of the gene encoding key raw-starch-degrading enzyme PoxGA15A. RsrC specifically binds rsrA promoter, but not amylase genes, to positively regulate the expression of rsrA and the production of raw starch-degrading enzymes. These findings expand complex regulatory network of fungal raw starch-degrading enzyme biosynthesis.
Similar content being viewed by others
Introduction
Amylase is an important industrial enzyme1, which can convert starch into glucose and/or maltose2,3. Traditional starch processing comprising of gelatinisation, liquefaction and saccharification, involves soluble starch-degrading enzyme, with all steps requiring high energy input4. In general, soluble starch-degrading enzymes are recognized as glycoside hydrolases degrading soluble or cooked starch. By contrast, raw starch-degrading enzymes are capable of directly degrading raw or uncooked starch granules into oligosaccharides or glucose below gelatinisation temperature5. Application of raw starch-degrading enzymes can improve production efficiency and reduce input costs and environmental pollution, giving them great market application prospects6.
Penicillium can secrete intact and highly active raw starch-degrading enzyme7, including raw starch-degrading glucoamylase PoxGA15A8. However, low production of native raw starch-degrading enzymes means they cannot meet the needs of large-scale industrial applications, hence it is urgent to elucidate the regulatory mechanisms of amylase gene expression to guide molecular breeding of fungal strains that produce large quantities of raw starch-degrading enzymes.
Expression of amylase genes in Penicillium oxalicum is controlled by a variety of regulatory factors. For instance, activator AmyR binds to the promoters of genes encoding prominent amylases including PoxGA15A and α-amylase Amy13A. AmyR interacts with histone acetyltransferase complex Hat1-Hat2, acting as a molecular brake to regulate expression of amylase genes9. Moreover, CxrC negatively regulates the expression of genes encoding major raw starch-degrading enzymes10. Other specific regulatory factors such as translational elongation factor eEF1A11, G protein γ subunit GNG-112, protein kinases PoxMK113, PoxMKK114 and POGSK‑3β15, GATA-type zinc finger protein NsdD16 and putative methyltransferase Mtr23B17, are known to participate in raw starch-degrading enzyme biosynthesis in P. oxalicum.
Previous studies identified a transcription factor (TF), RsrA (formerly POX01907), containing two SANT-like domains with different roles18. SANT1 is responsible for DNA-binding, while SANT2 interacts with a putative 3-hydroxyisobutyryl-CoA hydrolase. RsrA positively regulates the expression of PoxGA15A and amy13A, and this regulation is dependent on its phosphorylation and the recruitment of Mediator complex19. However, the regulatory circuit associated with RsrA remains unknown.
In present study, TFs RsrB and RsrC acting downstream and upstream of RsrA, respectively, were identified in P. oxalicum through comparative transcriptomic profiling and yeast one-hybridisation (Y1H). Both of them positively regulated the biosynthesis of raw starch-degrading enzymes and conidiation, constructing a regulatory circuit RsrC-RsrA-RsrB.
Results
POX_g08691 positively regulates amylase production of P. oxalicum
Comparative analysis of transcriptomes from P. oxalicum mutant ΔrsrA and parental strain Δku70 was performed following culture in medium containing commercial starch of corn for 4 h, and 15 differentially expressed genes (DEGs) encoding putative TFs were identified in ΔrsrA relative to Δku70, using thresholds of |Log2 fold change| >1.5 and p value < 0.0518. These thresholds were selected with aim to increase the probability of screening target genes. Of these, six DEGs were successfully deleted in Δku70 in previous reports20. Herein, deletion mutants of the remaining nine DEGs were constructed (Supplementary Fig. S1). Further measurement of raw starch-degrading enzyme activity revealed seven mutants with significantly altered enzyme production relative to Δku70. Notably, mutant ΔPOX_g08691 had the greatest reduction in enzyme production (49.5%) when directly cultured on commercial starch of corn for 6 days (Supplementary Fig. S2). Therefore, POX_g08691 was selected for further analysis.
Moreover, complementation strain CPOX_g08691 was constructed and confirmed (Supplementary Fig. S3), where the complementation cassette of POX_g08691 was introduced into an ectopic locus of POX_d05452 encoding aspartic protease PepA. Deletion of POX_d05452 did not affect the production of amylases by P. oxalicum21. Subsequently, it was found that production of raw starch-degrading enzymes and soluble starch-degrading enzymes by ΔPOX_g08691 was 58.4–63.0% lower than that by Δku70 when cultivated for 2 or 4 days. Complementation strain CPOX_g08691 partially restored amylase production compared with Δku70 (Fig. 1a, b). In addition, sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis indicated that the secreted extracellular proteins by ΔPOX_g08691 were lower than that by Δku70 when cultivated for 4 days. The extracellular proteins of CPOX_g08691 were comparable to those of Δku70 (Supplementary Fig. S3c). These results indicate that POX_g08691 positively regulates the biosynthesis of amylases in P. oxalicum.
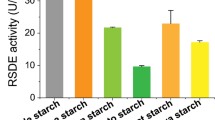
a Production of raw starch-degrading enzymes (RSDEs) and soluble starch-degrading enzymes (SSDEs) (b) by P. oxalicum mutant ΔrsrB, parental strain Δku70 and complementation strain CrsrB in the presence of soluble corn starch (SCS). Lowercase letters represent p < 0.05. Different letters indicate significant differences, evaluated by one-way ANOVA. c Conserved domains in RsrB. Grey and green areas indicate the Gal4-like Zn2Cys6 zinc finger domain (Gal4) and Fungal_TF_MHR domain, respectively. d Phylogenetic analysis of RsrB and its homologues. The cladogram was constructed by MEGA version X using the neighbour-joining method and a Poisson model. Values displayed on branches were calculated using 1000 bootstrap replicates. e Yeast self-activation assay. Yeast cells carrying different lengths of RsrB peptides were cultured on SDO (SD/-Trp) and SDO/X/A (SD-Trp/+ X-α-Gal /+ aureobasidin A) for 4 days. f Effects of rsrB overexpression on RSDE and SSDE (g) production of P. oxalicum. All tested P. oxalicum strains were cultured in medium containing soluble corn starch for 2–4 days after transfer from glucose. Each mutant included three independent transformants. Results are mean ± SD. All experiments were performed at least three times. Uppercase and lowercase letters represent p < 0.01 and p < 0.05, respectively. Different letters indicate significant differences, evaluated by one-way ANOVA.
RsrB is a TF containing a Zn2Cys6 zinc finger domain
Genome annotation of P. oxalicum HP7-122 indicated that POX_g08691 consisting of 809 amino acid residues, contained a Gal4-like Zn2Cys6 zinc finger domain and a Fungal_trans domain (Fig. 1c). POX_g08691 shares 98.9% identity with PDE_01952 (EPS27011.1) from P. oxalicum 114-2, but <50% identity with orthologs in other fungal strains. Evolutionary analysis indicated that POX_g08691 and its orthologs were similar in Penicillium and Aspergillus (Fig. 1d). To facilitate further study, POX_g08691 was re-designated RsrB (Production of raw-starch-degrading enzyme regulator B).
To determine whether RsrB has transcriptional activation ability, an autoactivation experiment was conducted in yeast Y2HGold cells. The full-length rsrB gene and a DNA fragment encoding polypeptides RsrB176–809, RsrB176–210 and RsrB211–809 were separately cloned into plasmid pGBKT7, and the resulting recombinant plasmids were introduced into yeast Y2HGold cells. Recombinant Y2HGold/pGBKT7-rsrB1–809, Y2HGold/pGBKT7-rsrB176–210 and Y2HGold/pGBKT7-rsrB211–809 grew normally on SDO (SD/-Trp) plates but not on SDO/X/A (SD/-Trp/+X-α-Gal/+aureobasidin A). By comparison, recombinant Y2HGold/pGBKT7-rsrB176–809 grew normally on both SDO and SDO/X/A plates. In addition, colonies of Y2HGold/pGBKT7-rsrB176–809 turned blue on SDO/X/A plates (Fig. 1e), indicating that RsrB176–809 has transcriptional activation activity.
RsrB affects mycelial growth and conidiation of P. oxalicum
To explore the effects of RsrB on colony growth and sporulation, ΔrsrB was cultivated on solid plates containing PDA, glucose and commercial starch of corn, and the parental strain Δku70 and complementation strain CrsrB served as controls. When cultured for 5 days, the diameter of ∆rsrB colonies was smaller than that of Δku70 and CrsrB on PDA, but larger than on glucose and commercial starch of corn, respectively (Supplementary Fig. S4a). Quantitative analysis of asexual spores revealed that mutant ΔrsrB production of asexual spores was increased by 63.0%, 49.0% and 49.0% compared with Δku70 on PDA, glucose, and commercial starch of corn, respectively (Supplementary Fig. S4b).
Investigation of mycelial growth affected by RsrB displayed that mycelial accumulation in ΔrsrB was enhanced by 17.2–43.2% and 17.2–28.8% in glucose and commercial starch of corn, respectively (Supplementary Fig. S4c, d). Under microscopy, the hyphae of ΔrsrB cultivated for 48 h produced conidiospores earlier than those of both Δku70 and CrsrB after cultivation on PDA, glucose and commercial starch of corn, whereas CrsrB conidiospores were similar to those of Δku70 (Supplementary Fig. S5).
Overexpression of rsrB markedly improves amylase production
Moreover, overexpression strain OrsrB was constructed (Supplementary Fig. S6). In OrsrB, the overexpression cassette of rsrB was introduced into an ectopic locus of POX_d0545221 in Δku70, where rsrB is controlled by its own promoter. Further RT-qPCR assay revealed that the transcriptional level of rsrB was considerably enhanced by 0.69–1.87-fold in OrsrB relative to Δku70 in the presence of commercial starch of corn for 12–48 h (Supplementary Fig. S6c). The production of raw starch-degrading enzymes and soluble starch-degrading enzymes by OrsrB was enhanced by 61.4–81.2% after induction of commercial starch of corn for 2–4 days (Fig. 1f, g). SDS-PAGE analysis displayed that the secreted extracellular proteins by OrsrB was enhanced than that by Δku70 when cultivated for 2 or 4 days (Supplementary Fig. S6d). However, colony phenotypes and sporulation of OrsrB on plates containing PDA, glucose and commercial starch of corn were similar to those of Δku70 (Supplementary Fig. S7).
RsrB regulates expression of genes encoding amylase and sporulation
To elucidate the effects of RsrB on expression of genes related to amylase production, RNA-sequencing (RNA-seq) was employed. Total RNAs of both ∆ku70 and ΔrsrB were collected following cultivation in commercial starch of corn medium for 24 h after pre-growth in glucose. Statistical analysis of sequencing data from three biological replicates yielded a high Pearson correlation coefficient (Supplementary Fig. S8a), suggesting that these transcriptomic data were suitable for subsequent analysis.
The clean reads obtained by RNA-seq were mapped onto the genome of P. oxalicum strain HP7-122, and the expressed genes were screening. Comparative analysis identified 4041 DEGs in ΔrsrB compared with ∆ku70, with a threshold False Discovery Rate (FDR) < 0.05, consisting of 2265 upregulated (0.08 <log2 fold change <8.5) and 1776 downregulated (-10.6 <log2 fold change <-0.08) genes (Fig. 2a and Supplementary Data 1). These DEGs were mainly involved in metabolism (651 genes) and genetic information processing (521 genes), especially carbohydrate metabolism (186 genes), and translation (272 genes; Fig. 2b).
a Volcano plot indicating differentially expressed genes (DEGs). DEGs were screened and detected with thresholds of 0 <False Discovery Rate (FDR) ≤ 0.05. b KEGG annotation of DEGs modulated by RsrB. c DEGs encoding amylases. d DEGs encoding putative sugar transporters. e DEGs encoding putative transcription factors. f Real-time reverse transcription quantitative PCR (RT-qPCR) analysis of genes encoding major amylases, as well as sporulation-regulatory gene brlA (g). All P. oxalicum strains were cultured for 4–24 h in the presence of SCS. Gene expression in ΔrsrB was normalised to the level of Δku70. Results are mean ± SD. **p < 0.01 and *p < 0.05 indicate significant differences between ΔrsrB and Δku70, calculated by Student’s t test. PoxGA15A, raw starch-degrading glucoamylase; POX_b02418, glucoamylase; Amy13A, α-amylase.
Among the 4041 DEGs, eight genes encoding amylolytic enzymes were identified, including key raw starch-degrading glucoamylase gene PoxGA15A, glucoamylase gene POX_b02418 and α-amylase gene amy13A, the expression of which was downregulated (-3.71 <log2 fold change <-1.88) in ΔrsrB compared with ∆ku70 (Fig. 2c). Forty-three DEGs encoding predicted sugar transporters were identified, of which nine were upregulated (0.63 <log2 fold change <1.31) and 34 were downregulated (-9.75 <log2 fold change <-0.44) in ΔrsrB. Three key cellulodextrin transporter genes cdtC, cdtD and cdtG were included, with downregulated transcription (Fig. 2d).
Moreover, 184 DEGs encoding putative TFs were identified, 84 of which were upregulated (0.18 <log2 fold change <4.53) and 100 were downregulated (-6.04 <log2 fold change <-0.26) in ΔrsrB. Of them, several known TF-encoding genes regulating the biosynthesis of RSDEs in P. oxalicum were found, such as cxrC/POX_a0054110 and amyR/POX_f080979, with 84.1%-increased and 47.0%-reduced expression in ΔrsrB. The transcription of the key sporulation-regulated POX_c03558/brlA gene increased 22.6-fold in ΔrsrB (Fig. 2e).
Two genes (POX_a00279/rodA-like and POX_c03497/rodB) that are involved in fungal mycelial growth and sporulation16 were markedly upregulated by 31.57- and 35.01-fold, respectively, following rsrB deletion.
Real-time quantitative reverse transcription PCR (RT-qPCR) assay found that expression of the PoxGA15A, POX_b02418, amy13A and brlA, varied in ΔrsrB compared with Δku70. After culturing for 4 h, transcription of PoxGA15A in ΔrsrB was increased 2.57-fold, whereas POX_b02418 and amy13A were downregulated by 20.8% and 67.5%, respectively. At both 12 and 24 h, transcription of PoxGA15A, POX_b02418 and amy13A genes was downregulated 51.3–90.6% (Fig. 2f). By comparison, at both 4, 12 and 24 h, transcription of brlA increased 0.94–3.98-fold (Fig. 2g).
RsrB binds to the promoter regions of amylase genes and brlA
In vitro electrophoretic mobility shift assay (EMSA) was performed to determine whether RsrB binds to the promoter regions of amylase genes PoxGA15A, POX_b02418 and amy13A. The results showed shifts in bands representing DNA-protein complexes when rRsrB165–271 was added to the probe tagged with 6-carboxyfluorescein (6-FAM), and the band density increased with an increasing amount of rRsrB165–271. As expected, neither control probe β-tubulin gene promoter nor proteins BSA and Trx-His-S formed DNA-protein complexes. Subsequent competitive EMSA indicated that the formed complexes markedly decreased or disappeared with increased competitive probes lacking 6-FAM (Fig. 3a, b). Similarly, a shifted band appeared when the mixture of rRsrB165–271 and brlA probe was loaded, but not when loading the competitive probe lacking 6-FAM (Fig. 3c). These results suggest that RsrB specifically binds to the promoter regions of the tested amylase genes and brlA.
a–c Each reaction contained Trx-His-S-tagged rRsrB165–271 (0–3 μg) and 6-carboxyfluorescein-tagged probe (~50 ng). Trx-His-S peptide and bovine serum albumen (BSA) served as controls, along with the promoter region of the β-tubulin gene. Competitive probes were DNA fragments without 6-carboxyfluorescein. PoxGA15A, raw starch-degrading glucoamylase gene; POX_b02418, glucoamylase gene; amy13A, α-amylase gene; brlA, conidiation regulatory gene. d Schematic diagram indicating truncated promoter region of PoxGA15A probes for in vitro EMSA. e, f In vitro EMSA between rRsrB165–271 and truncated probes of PoxGA15A. Each reaction contained Trx-His-S-tagged rRsrB165–271 (3 μg) and 6-carboxyfluorescein-tagged probe (~50 ng). g MEME-predicted conserved DNA sequence bound by rRsrB165–271. DNA sequences were from promoter regions of PoxGA15A, POX_b02418 and amy13A. h EMSA indicating the binding of mutated PoxGA15A probes by rRsrB165–271 (3 μg). Mutated PoxGA15A probe has ‘A’ instead of T and C at the 1st and 15th positions, as shown in panel D.
RsrB binds to core DNA sequence 5’-KBKWYSNRKNDVVBS-3’
To identify DNA sequences bound by rRsrB165–271, a set of truncated PoxGA15A probes was designed, used for in vitro EMSA. The PoxGA15A-1040–-53 probe was successively truncated when attached to the 3’-terminus, generating several DNA fragments (Fig. 3d). All truncated probes tagged with 6-FAM formed DNA-protein complexes with recombinant rRsrB165–271 except PoxGA15A-510–-53 (Fig. 3e). Similarly, when attached the 5’-terminus, the PoxGA15A-1040–-53 probe was truncated to different lengths of DNA fragments (Fig. 3d). In vitro EMSA showed that rRsrB165–271 could bind to all tested probes except PoxGA15A-1040–-498 (Fig. 3f). It therefore appears that the core-DNA sequence bound by rRsrB165-271 is PoxGA15A-511–-497 (5’-TCGATCAGGCACGCC-3’).
Analysis by the MEME suite (https://meme-suite.org/meme/) with promoter regions from PoxGA15A, POX_b02418 and amy13A identified the core DNA-binding sequence 5’-KBKWYSNRKNDVVBS-3’ (K: G/T; B: G/C/T; W: A/T; Y: C/T; S: G/C; N: G/A/C/T; R: A/G; D: G/A/T; V: G/A/C) shown in Fig. 3g. Based on the above results (Fig. 3e and f), two key nucleotides (T1 and C15) were localised at the binding site of rRsrB165-271 at the PoxGA15A promoter. When they were separately mutated to A, the shifted bands formed by rRsrB165-271 and the mutated probes PoxGA15AT1A or PoxGA15AC15A became weaker. When they were both mutated to A, the double mutated probe PoxGA15AT1A/C15A cannot be bound by rRsrB165-271 (Fig. 3h), suggesting that these nucleotides were essential for the target sequence to be bound by rRsrB165-271.
RsrB acts downstream of RsrA but with specific roles in regulation
Comparative transcriptomic analysis found that the transcript abundance of the rsrB gene significantly decreased by 67.8% in ΔrsrA relative to Δku7018. RT-qPCR analysis further confirmed that rsrB transcription decreased by 38.4%–76.9% after deletion of rsrA when cultivated in medium containing SCS for 4–48 h (Fig. 4a). In addition, in vitro EMSA indicated that recombinant rRsrA830-88319 formed a complex with 6-FAM-tagged rsrB probe, and the formed complex markedly decreased or disappeared when untagged competitive probe was loaded. The shifted bands did not appear when loading rRsrA830-883 and control β-tubulin gene probes, or between control protein BSA or Trx-His-S and tested probes (Fig. 4b). These results showed that RsrA specifically binds to the promoter region of rsrB.
a Real-time reverse transcription quantitative PCR (RT-qPCR) indicating expression of rsrB in mutant ΔrsrA on soluble corn starch (SCS). P. oxalicum strains were cultured for 4–48 h after transfer. mRNA levels in mutant ΔrsrA were normalised to the levels in parental strain Δku70 at the corresponding timepoints. **p < 0.01 and *p < 0.05 by Student’s t test represent significant differences between deletion mutant ΔrsrA and Δku70. Results are means ± SD. b In vitro EMSA between RsrA and rsrB probe. Recombinant rRsrA830–883 (0–3 μg) and rsrB probe (50 ng) were loaded. Trx-His-S peptide and bovine serum albumen (BSA) served as controls, along with the promoter region of the β-tubulin gene. Competitive probes were DNA fragments without 6-carboxyfluorescein. c MEME-predicted conserved DNA sequences bound by rRsrA830–883. DNA sequences were from the promoter regions of PoxGA15A, POX_b02418, amy13A and rsrB. d EMSA indicating the binding of mutated rsrB probes by rRsrA830–883 (3 μg). Mutated probes have ‘A’ or ‘T’ instead of ‘G at the 1st, 6th and 7th positions, as shown in (c). e EMSA between mutated rRsrA830–883 (3 μg) and rsrB probes. In mutated rRsrA830–883, R866 is exchanged for A or K. ‘–’ indicates no protein added. (f, g) Production of RSDEs and SSDEs by P. oxalicum parental strain Δku70, deletion mutants ΔrsrB and ΔrsrA, and double mutant ΔrsrBΔrsrA. These strains were cultured in medium containing SCS for 2–4 days after transfer from glucose. Each mutant included three independent transformants. Results are mean ± SD. All experiments were performed at least three times. Lowercase letters represent p < 0.05. Different letters indicate significant differences, evaluated by one-way ANOVA. RSDE raw starch-degrading enzyme, SSDE soluble starch-degrading enzyme.
Previous studies found that RsrA binds to the core DNA sequence 5’-RHCDDGGD-3’ (R: G/A; H: T/C/A; D: T/G/A)19. Analysis using the MEME suite indicated that rsrB-292–-285 (5’-GTATTGGA-3’) might be required for RsrA binding, and three nucleotides (G1, G6 and G7) were relatively conserved (Fig. 4c). When they were mutated to A, A and T, respectively, the mutated probes couldn’t be bound by RsrA (Fig. 4d), suggesting that these residues are essential for the target sequence to be bound by RsrA.
In addition, residue R866 in RsrA is required for binding DNA19. As expected, in vitro EMSA found a clear shifted band when loading rRsrA830–883 and rsrB probes, but not between mutated rRsrA830–883R866K and rsrB probe. Notably, a weak shifted band appeared when loading a mixture of mutated rRsrA830-883R866A and rsrB probe (Fig. 4e). These results show that residue R866 of RsrA is required for binding to the promoter region of rsrB.
Double deletion mutant ΔrsrBΔrsrA was successfully constructed (Supplementary Fig. S9), and its secreted raw starch-degrading enzyme production was 86.1% and 66.8% less than that of mutants ΔrsrB and ΔrsrA, respectively (Fig. 4f), and raw starch-degrading enzyme and soluble starch-degrading enzyme production was also diminished by 87.8% and 74.2% (Fig. 4g). SDS-PAGE analysis indicated that the extracellular proteins by ΔrsrBΔrsrA was lower than that by mutants ΔrsrB and ΔrsrA when cultivated for 2 or 4 days (Supplementary Fig. S9c).
When cultivated on solid plates containing PDA, glucose and commercial starch of corn, the colony diameter of mutant ΔrsrBΔrsrA was comparable to that of ∆rsrA but larger than that of Δku70 and ΔrsrB on PDA. However, the diameter of ΔrsrBΔrsrA was shorter than that of ΔrsrB on glucose and commercial starch of corn (Supplementary Fig. S4a). Moreover, number of asexual spores by ΔrsrBΔrsrA was similar to that of ∆rsrA, but reduced by 70.0–88.0% compared with ΔrsrB on PDA, glucose and commercial starch of corn (Supplementary Fig. S4b). Microscopy revealed that hyphae of mutant ΔrsrBΔrsrA were similar to ΔrsrA (Supplementary Fig. S5).
POX_a01508 specifically binds to the promoter region of rsrA
To screen for proteins binding to the promoter region of rsrA, a DNA fragment without autoactivation activity was required for Y1H assay. We artificially designed 12 different lengths of DNA fragments upstream of the rsrA start code ATG, namely R1 to R12 (Supplementary Fig. S10a,) and used them as baits for self-activation testing. The results showed that all tested yeast strains grew well on synthetic deficiency medium (SD)/-Ura. After adding aureobasidin A (AbA), growth of yeast strains Y1H/R11 and Y1H/R12 was gradually inhibited with an increasing amount of AbA, as was that of the Y1H/p53 strain, whereas the other Y1H/R1 to Y1H/R10 was almost unaffected. Furthermore, when AbA was added at 150 ng/mL or more, Y1H/R12 exhibited no growth, indicating that R12 did not have a self-activation effect and could be used for subsequent Y1H screening (Supplementary Fig. S10b).
A cDNA library comprising 287 putative TF genes from P. oxalicum strain HP7-1, in which cDNA fragments were integrated into plasmid pGADT7, was constructed for Y1H assay. These 287 recombinant plasmids were individually transformed into yeast strain Y1H/R12 to screen for interacting proteins. When cultured on both SD/-Leu and SD/-Leu/AbA for 3 days, only the Y1H strain containing R12 and pGADT7-POX_a01508 grew normally, consistent with positive strain Y1H/cbh1/pGADT7-cxrA. TF CxrA was demonstrated to specifically bind the promoter of cellobiohydrolase gene cbh123. The negative control Y1H/cbh1/ pGADT7 only grew normally on SD/-Leu, but not on SD/-Leu/AbA (Fig. 5a). Therefore, POX_a01508 likely binds specifically to the promoter region of rsrA.
a Y1H assay. Yeast cells carrying DNA fragments upstream of rsrA were cultured on SD/-Leu and SD/-Leu/AbA200 for 3 days. b Conserved domains in RsrC. Purple areas indicate Cys2His2 (C2H2)-type zinc finger domains. c Phylogenetic analysis of RsrC and its homologues. The cladogram was constructed by MEGA version X with the neighbour-joining method and a Poisson model. Values displayed on branches were calculated using 1000 bootstrap replicates. Yeast self-activation assay. Yeast cells carrying different lengths of RsrC peptides (d) were cultured on SDO (SD/-Trp) and SDO/X/A (SD-Trp/+ X-α-Gal /+ aureobasidin A) for 3 days (e).
RsrC is a C2H2-type TF
The gene POX_a01508 is 1329 bp in length, containing three introns and encoding a polypeptide with 335 amino acids. The POX_a01508 protein contains four Cys2His2 (C2H2)-type zinc finger domains based on SMART analysis online (Fig. 5b). Furthermore, POX_a01508 shared 100% sequence identity with PDE_08462 (EPS33500.1) in P. oxalicum strain 114-2, which has no known function, followed by PECM_003408 from Penicillium ucsense strain S1M29 with an identify of 88.72%. By contrast, POX_a01508 shares lower identity (<70%) with homologues from other filamentous fungi such as Fusarium, Aspergillus and Trichoderma. Phylogenetic tree analysis showed that POX_a01508 was closely related to orthologs in Penicillium and Aspergillus species, while distantly related to those in Trichoderma, Fusarium and Neurospora (Fig. 5c). For convenience in future research, POX_a01508 was re-named as RsrC (Production of raw-starch-degrading enzyme regulator C in Penicillium oxalicum).
To test the transcriptional activation activity of RsrC, seven peptides of different lengths, namely U1 to U7, were designed as shown in Fig. 5d. DNA fragments encoding U1 to U7 were ligated to linearised pGBKT7 vector. The resulting recombinant plasmids were transformed into yeast Y2HGold competent cells. The obtained transformants were plated on SDO and SDO/X/A and incubated at 30 °C for 3 days. All colonies grew normally on SDO, indicating successful introduction of plasmids into Y2HGold cells. Strains Y2H/rsrC, Y2H/U1, Y2H/U2, Y2H/U3 and Y2H/U4 did not grow on SDO/X/A, consistent with the negative control. On the other hand, strains Y2H/U5, Y2H/U6 and Y2H/U7 grew normally and turned blue (Fig. 5e), indicating that U5, U6, and U7 can activate transcription of reporter genes AUR1-C and MEL1 in the Y2HGold cells, demonstrating a transcriptional activation function.
RsrC positively regulates amylase production
To explore the role of RsrC in amylase biosynthesis in P. oxalicum, deletion mutant ΔrsrC was constructed and confirmed by PCR (Supplementary Fig. S11a, b). Compared with Δku70, mutant ΔrsrC exhibited a significant decrease in production of raw starch-degrading enzymes and soluble starch-degrading enzymes, ranging from 79.0% to 85.9% (Fig. 6a, b). Enzyme production by CrsrC (Supplementary Fig. S11c and d) was comparable to that by Δku70 after 2 days of induction, but considerably enhanced at 4 days (Fig. 6a, b).
a Production of RSDEs and SSDEs (b) by P. oxalicum mutant ΔrsrC, parental strain Δku70, complementation strain CrsrC and overexpression strain OrsrC in the presence of SCS. Lowercase letters represent p < 0.05. Different letters indicate significant differences, evaluated by one-way ANOVA. c Colony observation of P. oxalicum mutant ΔrsrC, parental strain Δku70, complementation strain CrsrC and overexpression strain OrsrC on PDA, glucose and SCS plates for 4–5 days.
Moreover, the rsrC DNA cassette was integrated into the genome of P. oxalicum strain Δku70 at the POX_d05452 gene locus to generate overexpression strain OrsrC, in which expression of rsrC was activated by constitutive promoter Ptef1 (Supplementary Fig. S11e, f). Further RT-qPCR assay revealed that the transcriptional level of rsrC was considerably enhanced by 0.38–2.33-fold in OrsrC relative to Δku70 (Supplementary Fig. S11g). When cultivated on commercial starch of corn for 4 days, raw starch-degrading enzyme and soluble starch-degrading enzyme production of overexpression strain OrsrC markedly increased by 90.2% and 76.3%, respectively, whereas at 2 days enzyme production decreased by 54.2% and 69.6% (Fig. 6a, b). SDS-PAGE analysis showed that mutant ΔrsrC secreted less extracellular proteins than the Δku70 when cultivated for 2 or 4 days, whereas the overexpression strain OrsrC secreted more extracellular proteins (Supplementary Fig. S11h). These results suggest that RsrC positively regulates the production of amylase in P. oxalicum, especially during the later stages of induction.
RsrC affects mycelial growth of P. oxalicum
When inoculated onto solid plates containing PDA, glucose and commercial starch of corn as carbon sources, and incubated for 4–5 days, the colonies of ΔrsrC exhibited colour changes to various degrees, and they were considerably smaller than those of Δku70 or CrsrC. Comparatively, overexpression of rsrC also caused a change of colony colour, but colonies became larger. However, the colony colour and size of complemented strain CrsrC were partially restored to those of Δku70 (Fig. 6c).
RsrC widely regulates gene expression on commercial starch of corn
Both parent Δku70 and mutant ΔrsrC were separately cultured in medium containing commercial starch of corn for 24 h after transfer, and total RNAs were extracted and used for transcriptome sequencing analysis. The vales ranging from 0.97 to 0.99 for each sample (Supplementary Fig. S8b), indicating that the transcriptome data were reliable and could be used for further data analysis.
Gene expression levels were compared between mutant ΔrsrC and parental strain Δku70, using p value < 0.05 as the screening criterion. Based on the annotation of genome of P. oxalicum strain HP7-122, in mutant ΔrsrC, 4137 genes showed marked differences in expression relative to those in Δku70 (Supplementary Data 2). Among these genes, 2059 were significantly upregulated (0.23 <Log2Fold change <8.61), while 2078 were significantly downregulated (-10.2 <Log2Fold change <-0.22; Fig. 7a). These DEGs encoded proteins mainly involved in metabolic processes, particularly carbohydrate metabolism (accounting for 16.7%) and amino acid metabolism (13.5%), followed by genetic information processes including translation (10.9%; Fig. 7b).
a Volcano plot showing DEGs. DEGs were selected with threshold p ≤ 0.05. b KEGG annotations of DEGs modulated by RsrC. c DEGs encoding carbohydrate-active enzymes (CAZymes). d DEGs encoding amylolytic genes. e DEGs encoding putative transcription factors. f DEGs encoding factors related to gene transcription. g RT-qPCR analysis of genes encoding major amylases, as well as key regulatory gene rsrA (h). All P. oxalicum strains were cultured for 12–48 h in the presence of SCS. Gene expression in ΔrsrC was normalised to the level of Δku70. Results are mean ± SD. **p < 0.01 and *p < 0.05 indicate significant differences between ΔrsrC and Δku70, calculated by Student’s t test. PoxGA15A, raw starch-degrading glucoamylase; POX_b02418, glucoamylase.
Among the DEG regulon of rsrC, 345 DEGs encoding carbohydrate-active enzymes (CAZymes) were detected in ΔrsrC, including 189 upregulated (0.24 <Log2Fold change <4.03) and 156 downregulated (-4.04 <Log2Fold change <-0.22) genes. These DEG-encoding proteins that could be classified into six CAZyme families, namely glycosyltransferase (GT; 24.63%), auxiliary oxidoreductase (AA; 15.65%), glycoside hydrolase (GH; 41.45%), carbohydrate esterase (CE; 14.49%), carbohydrate-binding module (CBM 11.30%) and polysaccharide lyase (PL; 2.31%; Fig. 7c). Interestingly, among these DEGs encoding glycoside hydrolases, six genes encoding amylolytic enzymes were found in mutant ΔrsrC relative to the parental strain Δku70, including PoxGA15A, POX_b02418, 1,4-α-glucan branching enzyme gene POX_d05520, and three α-glucosidase genes POX_f08248, POX_c03853 and POX_e06687. The transcriptional levels of three genes decreased in ΔrsrC; PoxGA15A by 49.7%, POX_b02418 by 63.7% and POX_e06687 by 55.2% (Fig. 7d).
Additionally, there were 225 genes encoding specific TFs, most containing conserved zinc finger domains (146), followed by winged helix repressor DNA-binding domains (27). Among these, 62 genes showed upregulation (0.25 <Log2 fold change <4.13), while 163 genes showed downregulation in ΔrsrC (-10.16 <Log2 fold change <-0.28). Notably, four known regulatory genes (creA/POX_e0719224, rsrA/POX_a00019 and rsrB) were identified, and the former one repressed expression of amylase genes, whereas the latter two activated expression18. Expression of both rsrA and creA in ΔrsrC was downregulated by 23.2% and 27.7%, respectively, whereas rsrB were upregulated by 26.2% (Fig. 7e).
In addition to the specific TFs, other factors relative to gene transcription, such as RNA polymerase complex, basal TFs, and the spliceosome complex25, were screened from proteins encoded by DEGs in the rsrC regulon. The results revealed 71 relative proteins including 14 RNA polymerase subunits (including RNA polymerase I subunit RPA1, RPA12 and RPA12; RNA polymerase II subunit RPB1 and RPB7, RNA polymerase III subunits RPC1, RPC2, RPC3, RPC7 and RPC11, RNA polymerases I and III subunits RPAC1 and RPAC2; RNA polymerases I, II, and III subunits RPABC1 and RPABC3), 11 basal TFs (including transcription initiation factor TFIID subunits TAF1, TAF2, TAF3, TAF6, TAF7, TAF9, TAF12 and TAP), as well as 40 spliceosome-relative proteins including pre-mRNA-processing factors PRP8, PRP19, PRP43, SLU7 and SLT11; and U4/U6 small nuclear ribonucleoprotein PRP3, PRP4 and PRP31. More than 60% of these were downregulated in ΔrsrC, especially RNA polymerase subunits (Fig. 7f).
Absorption of sugars by fungal cells is dependent on sugar transporters. Screening annotation of 4137 DEGs revealed 36 genes encoding sugar transporters, and comparative analysis identified 14 genes that were significantly upregulated (0.42 <Log2 fold change <3.85) in ΔrsrC, while 22 were significantly downregulated (-5.46 <Log2Fold change <-0.37). Notably, the predominant cellulose dextrin transporter gene cdtC was downregulated by 58.8% (Supplementary Data 2).
Moreover, RT-qPCR analysis was employed to confirm the results from comparative transcriptomics. The results showed that, compared with parental strain Δku70, the expression level of PoxGA15A in ΔrsrC was considerably downregulated, by 75.6% and 42.8% after 12 and 24 h of cultivation, respectively. Additionally, the expression level of POX_b02418 was downregulated by 58.9%, 69.3% and 26.9% after 12, 24 and 48 h of cultivation, respectively (Fig. 7g). As expectedly, expression of rsrA decreased by 47.3–90.9% in ΔrsrC (Fig. 7h). The results at 24 h were consistent with data from comparative transcriptomics.
RsrC positively regulates rsrA expression to promote amylase production
To investigate the regulatory relationship between RsrA and RsrC concerning the amylase production in P. oxalicum, mutant OrsrAΔrsrC was constructed and confirmed by PCR (Supplementary Fig. S12a, b), using OrsrA as the background strain. In the OrsrA, the expression of rsrA inserted was promoted by the constitutive promoter Ptef1, and native rsrA transcription was controlled by its own promoter19. Compared with control strain OrsrA, OrsrAΔrsrC exhibited a significant decrease of 56.2–60.1% in the production of raw starch-degrading enzymes and soluble starch-degrading enzymes, when cultured in commercial starch of corn medium for 2–4 days after transfer. Conversely, compared with ΔrsrC, there was a significant increase of 1.2–7.1-fold (Fig. 8a, b). SDS-PAGE analysis showed that the secreted extracellular proteins by OrsrAΔrsrC decreased in comparison with the control strain OrsrA when cultivated for 2 or 4 days (Supplementary Fig. S12e). This suggests that RsrC positively regulates the expression of RsrA to promote the amylase production.
a, b Production of RSDEs and SSDEs by P. oxalicum parental strain Δku70, deletion mutants ΔrsrC and ΔrsrA, overexpression strain OrsrA, mutant OrsrAΔrsrC, and double mutant ΔrsrAΔrsrC. These strains were cultured in medium containing soluble corn starch for 2–4 days after transfer from glucose. Each mutant included three independent transformants. Results are mean ± SD. All experiments were performed at least three times. Lowercase letters represent p < 0.05. Different letters indicate significant differences, evaluated by one-way ANOVA. RSDE, raw starch-degrading enzyme; SSDE, soluble starch-degrading enzyme. c Schematic diagram indicating the truncated promoter region of rsrA for Y1H assay. d Y1H assay between RsrC and the truncated region of upstream DNA sequence of rsrA. Yeast cells carrying DNA fragments upstream of rsrA are cultured on SD/-Leu and SD/-Leu/AbA200 for 3 days.
In addition, double mutant ΔrsrAΔrsrC was also successfully constructed (Supplementary Fig. S12c, d). Analysis of enzymatic activity revealed that during 2–4 days on commercial starch of corn, ΔrsrAΔrsrC showed a considerably decrease in production of raw starch-degrading enzymes and soluble starch-degrading enzymes compared with all control strains on day 2, and even produced negligible amylase on day 4 (Fig. 8a, b). SDS-PAGE analysis indicated that the ΔrsrAΔrsrC secreted reduced extracellular proteins compared with all control strains when cultivated for 2 or 4 days (Supplementary Fig. S12e). These results indicate that RsrA and RsrC specifically regulate the biosynthesis of amylase, and their regulation clearly overlaps.
Residues -850 to -825 bp upstream of rsrA are required for RsrC binding
To identify the core DNA sequence in the promoter region of rsrA that was bound by RsrC, a series of truncated DNA fragments, R13 to R18, were designed as baits for Y1H assay (Fig. 8c). Recombinant plasmid pGADT7-rsrC and control pGADT7 were separately transformed into yeast bait strains Y1H/R13, Y1H/R15, Y1H/R17 and Y1H/R18, and plasmid pGADT7-cxrA was transformed into Y1H/cbh1 as a positive control. All obtained yeast transformants were able to grow normally on SD/-Leu, indicating successful transformation of plasmids into bait strains. By comparison, on SD/-Leu/AbA200, Y1H/R13/pGADT7-rsrC, Y1H/R15/pGADT7-rsrC and Y1H/R17/pGADT7-rsrC showed normal growth, as did positive control Y1H/cbh1/pGADT7-cxrA, whereas Y1H/R18/pGADT7-rsrC and the Y1H/R13/pGADT7, Y1H/R15/pGADT7 and Y1H/R17/pGADT7 control strains could not grow (Fig. 8d). These results indicate that RsrC can bind to three DNA fragments (residues -1200 to -800, -900 to -800, and -850 to -800 bp) in the promoter region of rsrA, but not -825 to -800 bp, meaning that residues -850 to -825 bp are required for binding by RsrC.
Comparative analysis of regulons of RsrA, RsrB and RsrC
To further investigate similarities and differences between the regulation of RsrA, RsrB and RsrC in P. oxalicum, we comparatively analysed their regulons under induction after 24 h by commercial starch of corn. Transcriptome profiling of mutant ΔrsrA in the presence of commercial starch of corn was assessed by RNA-seq. Sequencing data from three biological replicates of each fungal strain were evaluated statistically and yielded a high Pearson correlation coefficient (Supplementary Fig. S8a).
With thresholds of FDR < 0.05, there were 5656 DEGs in ΔrsrA compared with ∆ku70, including 1438 upregulated and 4218 downregulated genes, named the RsrA regulon (Supplementary Data 3). Of these, only gene amy13A encoding the key α-amylase was downregulated (log2 fold change = -2.53). There were 305 putative TF-encoding genes, 51 of which were upregulated and 254 were downregulated. Notably, the key regulatory genes amyR and rsrC were found, with 30.0%- and 60.06%-reduced expression in the ΔrsrA. Moreover, 50 putative sugar transporter-encoding genes were detected, seven of which were upregulated and 43 were downregulated.
Comparative analysis of RsrB and RsrA regulons identified 2677 DEGs co-regulated (Fig. 9a), of which 663 were upregulated and 1272 were downregulated in both ΔrsrB and ΔrsrA, relative to those in ∆ku70 (Supplementary Fig. S13a). These co-regulated genes were mainly involved in metabolism and genetic information processing, especially carbohydrate, amino acid metabolism and translation (Supplementary Fig. S13b). Notably, the key amy13A was co-regulated, and its expression was downregulated (log2 fold change = -3.10 and -2.53) in ΔrsrB and ΔrsrA, respectively (Fig. 9b and Supplementary Fig. S13c). There were 125 co-regulated DEGs encoding TFs, 20 of which were upregulated and 74 were downregulated in both ΔrsrB and ΔrsrA (Fig. 9c and Supplementary Fig. S13d). Moreover, 33 co-regulated DEGs encoding putative sugar transporters were found, 26 of which were downregulated in both ΔrsrB and ΔrsrA (Fig. 9c and Supplementary Fig. S13e).
By contrast, 2741 DEGs co-regulated by RsrA and RsrC were detected (Fig. 9a), 938 of which were downregulated in both ΔrsrA and ΔrsrC compared with those in Δku70, while 425 were upregulated (Supplementary Fig. S14a). These co-regulated DEGs mainly participated in carbohydrate metabolism and translation (Supplementary Fig. S14b). Notably, 79 DEGs involved in ribosome biogenesis and assembly detected, of which 53 were upregulated in both ΔrsrA and ΔrsrC (Supplementary Fig. S14c). However, only three α-glucosidase genes POX_c03853, POX_e06687 and POX_g08885, and one 1,4-α-glucan branching enzyme gene POX_d05520 were included. Transcription of POX_e06687 decreased (-3.11 <log2 fold change <-1.16) in both ΔrsrA and ΔrsrC, whereas expression of POX_c03853 decreased (log2 fold change = -2.23) in ΔrsrA and increased (log2 fold change = 0.29) in ΔrsrC, expression of POX_d05520 and POX_g08885 decreased (-1.13 <log2 fold change <-0.80) in ΔrsrA but increased (0.31 <log2 fold change <1.38) in ΔrsrC (Fig. 9c and Supplementary Fig. S14d).
There were 2070 DEGs co-controlled by both RsrB and RsrC (Fig. 9a), including 401 upregulated and 307 downregulated genes in both ΔrsrB and ΔrsrC relative to those in Δku70 (Supplementary Fig. S15a). These co-regulated genes were mainly involved in translation and carbohydrate metabolism (Supplementary Fig. S15b). Notably, two amylase genes, PoxGA15A and POX_b02418, were co-regulated, and their expression was downregulated in ΔrsrB and ΔrsrC (Fig. 9c and Supplementary Fig. S15c). In addition, among the 2070 DEGs, 96 TF-encoding genes (Fig. 9c and Supplementary Fig. S15d) and 23 sugar transporter-encoding genes (Fig. 9c and Supplementary Fig. S15e) were identified.
Comparative analysis of the three regulons descried above identified 1408 DEGs co-regulated by RsrA, RsrB and RsrC, of which 180 were upregulated and 212 were downregulated in ΔrsrA, ΔrsrB and ΔrsrC, relative to ∆ku70 (Fig. 9a). These co-regulated genes were mainly involved in translation and carbohydrate metabolism (Fig. 9b). Among the 1408 DEGs, no gene encoding key amylases were detected. 69 co-regulated DEGs encoding TFs were identified. Of these, POX_e06829, POX_g08739 and POX_g08892 were upregulated and 24 were downregulated in ΔrsrA, ΔrsrB and ΔrsrC (Fig. 9c).
Discussion
In this study, we identified two TFs, RsrB and RsrC, acting downstream and upstream of RsrA, respectively, in P. oxalicum. RsrA is known to activate the expression of raw starch-degrading glucoamylase gene PoxGA15A and α-amylase gene amy13A18,19. RsrB and RsrC were found to regulate the biosynthesis of amylases including raw starch-degrading enzymes in the presence of commercial starch of corn, as well as conidiation and mycelial growth. Furthermore, RsrC stimulates the transcription of rsrA by directly binding to residues -850 to -800 bp in the promoter region of rsrA. RsrB can bind to the promoter regions of genes related to the biosynthesis of amylases and sporulation, thereby controlling their expression (Fig.10a). These findings enrich and expand the regulatory network of the expression of amylase genes.
Y2H assay indicated that RsrB176–809 has transcriptional activation ability, whereas RsrB176–210 and RsrB211–809 does not. RsrB176–210 is predicted to be the Gal4-like Zn2Cys6-type DNA-binding domain. In yeast, Gal4 contains two independently functional domains: an N-terminal DNA-binding domain and a C-terminal transcriptional activation domain26. However, RsrB176–210 is required for both DNA-binding and transcriptional activation ability. In addition, residues 132–335 in RsrC exhibited transcriptional activation activity. However, the full-length protein RsrB1–809 as well as RsrC1–335 do not demonstrate the transcriptional activation activity. The reason for these may be because protein folding and tertiary structure are different between yeast and P. oxalicum.
RsrA controls the expression of PoxGA15A and amy13A by directly binding to their promoter regions, which depends on R886. Notably, site-mutated R866A and R866K displayed different effects on raw starch-degrading enzyme production18,19. R866 was found to be required for RsrA binding to the rsrB promoter. Surprisingly, R866K lost the ability to bind rsrB probe, whereas R866A retained weak binding, but the reason is unknown. These differences in binding may explain why raw starch-degrading enzyme production by the R866A mutant was higher than that of R866K19.
Moreover, RsrA directly regulates the expression of rsrB, but RsrB cannot regulate the expression of rsrA. Double mutant ΔrsrBΔrsrA exhibited lower amylase production than individual ΔrsrB and ΔrsrA strains. Additionally, the diameter of ∆rsrB colonies is smaller than that of control strain Δku70, but colonies of ∆rsrA were larger than that of Δku70 on PDA. The ∆rsrB strain produced more asexual spores than Δku70, but this decreased for ∆rsrA. These results suggest that RsrA and RsrB have some overlap in the regulation of amylase biosynthesis and mycelial growth, but their independent regulatory pathways are not yet known, as confirmed by comparative analysis of their regulons. For example, there were 5656 DEGs in the rsrA regulon but only 4041 in the rsrB regulon, and 2677 were co-regulated by both RsrB and RsrA, including amy13A. However, RsrB regulated the expression of brlA and POX_c03497/rodB that participate in fungal growth and sporulation16, but RsrA did not.
Analysis of amino acid sequences identified RsrC as a C2H2-type zinc finger TF. In the literature, there are two other TFs containing a C2H2-type zinc finger domain, namely CreA24 and CxrB27, which participate in the biosynthesis of amylases in P. oxalicum. Interestingly, CreA mediates carbon catabolite repression, and suppresses the expression of amylase genes, whereas CxrB positively regulates the transcription of amylase genes. Additionally, CreA functions in both pathways, by directly binding to the promoter regions of genes encoding major RSDEs, and indirectly controlling the expression of other regulatory genes such as amyR24. CxrB indirectly controls the expression of amylases by directly regulating the transcription of amyR27. AmyR is known to be positively involved in amylase biosynthesis in fungi24. In addition, comparative transcriptomic analysis found that expression of creA was downregulated in ΔrsrC, whereas rsrB were upregulated. Therefore, RsrC controls the production of amylases via diverse cascade regulation. For instance, RsrC affects the expression of amylase genes, possibly by stimulating the expression of rsrA and thereby activating the transcription of amyR and rsrB; or activating the expression of creA, which inhibits the expression of amylase genes and other regulatory genes. Interestingly, comparative transcriptomics also revealed that the expression of rsrC is activated by rsrA (Fig. 10b). However, these regulations still require to be further confirmed through biochemical and genetic methods. Certainly, the final expression level of amylases gene in P. oxalicum depends on the balance of the regulatory network. Surprisingly, expression of rsrB was enhanced in ΔrsrC, suggesting that CreA may repress the expression of rsrB in the presence of commercial starch of corn.
Notably, the production of amylases by mutant OrsrAΔrsrC, in which the additionally inserted rsrA is under the control of constitute promoter Ptef1, could partially restore the altered enzyme production caused by rsrC deletion. This indicates that RsrC regulation in the production of raw starch-degrading enzymes involves both RsrA-dependent and RsrA-independent pathways. In addition, double mutant ΔrsrAΔrsrC exhibited considerably lower enzyme production than individual mutants ΔrsrA and ΔrsrC, suggesting that RsrA functions also involve RsrC-independent pathways. Furthermore, comparative analysis between RsrA and RsrC regulons revealed that the transcriptional abundance of genes PoxGA15A and amy13A was altered to different degrees in ΔrsrA and ΔrsrC relative to Δku70.
It should be noted that the promoter region of rsrA taken to identify the TF-binding site was very upstream, i.e., -800 to -1318. It is possible that the sites of some other important TFs might lie in the -1 to -800 region and it would be missed in this experiment. Notably, the region from -1 to -800 exhibited autoactivation activity, which was not suitable for Y1H assay.
Moreover, the production of raw starch-degrading enzymes and soluble starch-degrading enzymes by complementation strain CrsrC was considerably higher than that of parental strain Δku70 when cultured in medium containing commercial starch of corn for 4 days. The CrsrB (CPOX_g08691) did not fully complement the phenotypes resulting from deletion of rsrB. Those might be due to different effects caused by the alternative locus (POX_d05452) integrated via the complementation cassette22.
Molecular breeding of fungi through synthetic biology is an efficient strategy to improve the production of proteins such as raw starch-degrading enzymes28. Overexpression of rsrC and rsrB markedly improved the production of amylases in P. oxalicum, indicating that RsrC and RsrB are good molecular targets for genetic engineering to enhance fungal enzyme production. It was recently confirmed that overexpression of rsrA could enhance amylase production19. Therefore, synergistic effect among RsrA, RsrB and RsrC should be considered in molecular breeding.
Materials and methods
P. oxalicum strains and culture conditions
All P. oxalicum strains used in this study are listed in Supplementary Table S1. P. oxalicum strains were cultured on PDA plates for 5 days at 28 °C to generate asexual spores. All asexual spores were washed with Tween 80 (Sangon Biotech Co., Ltd., Shanghai, China) and precultured in modified minimal medium containing glucose for 24 h. Approximately 1 g of mycelia was collected, transferred to modified minimal medium containing commercial starch of corn (Sigma-Aldrich, St. Louis, MO, USA), and cultured for 2–4 days to produce crude enzyme used for measurement of enzymatic production, or cultured for 4–48 h after transfer for RNA-seq and RT-qPCR assay.
Cultivation of Saccharomyces cerevisiae strains was conducted on yeast extract peptone dextrose for preservation and/or reproduction. Synthetic deficiency (SD) media without different nutrients was used for yeast autoactivation experiments and Y1H assay.
Total DNA and RNA extraction from P. oxalicum
Mycelia of P. oxalicum were fractured by liquid nitrogen, dissolved in DNA extraction buffer for 15 min, and phenol-chloroform (phenol, Solarbio Life Sciences, Beijing, China; chloroform, Kelon Chemicals Co. Ltd., Chengdu, China) was used for protein removal. DNA was precipitated by anhydrous ethanol (Tianjin Fuyu Fine Chemicals Co., Ltd., Tianjin, China). Total RNA extraction from P. oxalicum was conducted using an RNAsimple Total RNA Kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s instructions.
Yeast autoactivation experiment
DNA fragments were amplified by PCR with specific primers (Supplementary Table S2) using Δku70 cDNA as template, then subcloned into vector pGBKT7 (TaKaRa, Dalian, China) using restriction enzymes EcoR1 and BamH1. Recombinant plasmids were transformed into the S. cerevisiae Y2HGold strain (TaKaRa) and transformants were cultured on SDO and SDO/X/A plates at 30 °C for 4 days. The final concentrations of aureobasidin A and X-α-gal added to SDO/X/A were 100 ng/mL and 0.04 mg/mL, respectively. The transformants tested on SDO and SDO/X/A plates yielded white and blue colonies, respectively, indicating the tested protein/peptide had transcriptional activation activity.
Y1H assay
One-to-one Y1H assay was used for screening TFs binding the rsrA promoter in accordance with the instructions provided with the Matchmaker Gold Yeast One-Hybrid System (TaKaRa). Both recombinant pAbAi and pGADT7-AD were co-transformed into Y1H Gold cells and positive transformants were selected on plates containing SD/-Leu medium with AbA (200 ng/mL). Meanwhile, the Y1H Gold strain carrying pAbAi-cbh1 and pGADT7-PoxCxrA served as a positive control.
RNA-seq analyses
Total RNA of P. oxalicum was sequenced on the BGI-SEQ-500 platform at BGI (Shenzhen, China). Acquired data were further analysed by BWA software version 0.7.10-r789 and Bowtie2 version 2.1.029. RSEM software version 1.2.1230 and NOISeq tool31 were also employed.
RT-qPCR assay
Total RNA of P. oxalicum was converted to single-stranded cDNA by HiScript III RT SuperMix for qPCR plus gDNA wiper (Vazyme Biotech Co, Ltd., Nanjing, China). Mixtures comprising cDNA used as template, primers corresponding to genes, and ChamQTM Universal SYBR qPCR Master Mix (Vazyme Biotech Co, Ltd.) were subjected to PCR amplification on a 7500 Real Time PCR System. The relative expression of each detected gene was analysed using the 2−ΔΔCT method32.
Construction of P. oxalicum mutants
DNA fragments were obtained via PCR amplification with specific primers (Supplementary Table S2) and purified using DNA purification Kit (Tiangen Biotech Co., Ltd., Beijing, China). The knockout cassette was generated by fusion PCR and introduced into P. oxalicum protoplasts by the PEG chemical method19. Transformants were screened using the antibiotic bleomycin (100 μg/mL) and/or G418 (800 μg/mL), then verified by PCR with specific primers (Supplementary Table S2).
Measurement of raw starch-degrading enzyme and soluble starch-degrading enzyme activity
Activities of raw starch-degrading enzyme and soluble starch-degrading enzyme of culture supernatant from P. oxalicum were tested using the 3,5-dinitrosalicylic acid method33. Briefly, raw cassava flour from a farmer’s market in Nanning (China) and commercial starch of corn (Sigma-Aldrich) was used for substrates to determine raw starch-degrading enzyme and soluble starch-degrading enzyme activities, respectively. Culture supernatant of 50 μL was mixed with the citrate phosphate buffer of 450 μL (pH 4.5) containing 1% (w/v) substrate, and the mixture reacted at 65 °C and 55 °C for 30 min, respectively. The inactivated crude enzyme was used as control. The released reducing sugar was measured with 3,5-dinitrosalicylic acid. One unit (U) of RSDE and SSDE activity was defined as the amount of enzyme that produced 1 μmol of reducing sugar per min from the appropriate substrate. The production of RSDE and SSDE was recorded as U/gram of dry mycelial weight.
Determination of P. oxalicum biomass
Fresh asexual spores (1 × 108) of P. oxalicum were inoculated into liquid media containing glucose and commercial starch of corn, then cultivated for 3 days at 28 °C with shaking at 180 rpm. The resulting mycelia were collected every 12 h, dried at 50 °C, and weighted.
Observation of P. oxalicum colony phenotype and mycelial development
Fresh asexual spores (1×108) of P. oxalicum were inoculated onto solid plates containing PDA, glucose and SCS, and cultivated at 28 °C for between 48 h and 5 days. A Canon EOS 6D camera (Canon Inc., Tokyo, Japan) and an Olympus DP480 light microscope (Olympus, Tokyo, Japan) were respectively used to take photographs of P. oxalicum colony phenotype and mycelial development. Images were analysed by cellSence Dimension digital imaging software (Olympus).
Heterologous expression of recombinant polypeptides and in vitro EMSA
Target DNA fragments were amplified by PCR with corresponding primer pairs (Supplementary Table S2) and products were cloned into plasmid pET-32a(+) to generate recombinant plasmids. Recombinant plasmids were introduced into freshly competent Escherichia coli Rossetta cells (TaKaRa) to obtain positive transformants which were screened using kanamycin antibiotic (50 µg/mL). E. coli cells were cultured in Luria-Bertani medium for 5 h at 30 °C, 1 mM isopropyl-β-d-thiogalactopyranoside (Solarbio Life Sciences, Beijing, China) was added to induce target protein expression, and culture was continued for 24 h at 16 °C. E. coli Rossetta cells were collected and disrupted to extract recombinant polypeptides, which were purified using ProteinIso Ni-NTA Resin (TransGen, Shanghai, China).
The procedure for in vitro EMSA was performed as described previously19. Probes of detected genes were amplified by PCR using corresponding primer pairs (Supplementary Table S2) and mixed with recombinant polypeptides for 20 min at 28 °C. Mixtures were passed through non-denatured polyacrylamide adhesive and band shifts were recorded using a Bio-Rad ChemiDoc MP Imaging System (Bio-Rad Laboratories, Hercules, CA, USA).
Bioinformatics software
Homologous alignment and evolution analyses were performed using MUSCLE and MEGA version X32, respectively.
Statistics and reproducibility
Statistical analysis of experimental data was performed using Microsoft Excel (Office 2019, Microsoft, Redmond, WA) and SPSS (IBM, Armonk, NY) with Student’s t test and one-way analysis of variance (ANOVA). Results were generated from at least three independent experiments and reproducibility was confirmed. Data values indicate mean ± standard deviation, where N equals the number of independent experiments.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data for gene sequences could be found in the submitted genome of P. oxalicum strain HP7-1 in GenBank (accession number JRVD02000000). Transcriptomic data for P. oxalicum strains have been deposited in the Sequence Read Archive database (Accession No. GSE245046 and GSE248520). All uncropped and unedited blots/gels were found in Supplementary Fig. S16. The numerical source data behind the graphs can be found in Supplementary Data 4.
References
Kikani, B. A. & Singh, S. P. Amylases from thermophilic bacteria: structure and function relationship. Crit. Rev. Biotechnol 42, 325–341 (2022).
Wang, Y. C. et al. High-level expression of a novel α-amylase from Thermomyces dupontii in Pichia pastoris and its application in maltose syrup production. Int. J. Biol. Macromol 127, 683–692 (2019).
Paul, J. S., Gupta, N., Beliya, E., Tiwari, S. & Jadhav, S. K. Aspects and recent trends in microbial α-amylase: a review. Appl. Biochem. Biotechnol 193, 2649–26981 (2021).
Elyasi Far, B., Ahmadi, Y., Yari Khosroshahi, A. & Dilmaghani, A. Microbial alpha-amylase production: Progress, challenges and perspectives. Adv. Pharm. Bull 10, 350–358 (2020).
Gu, L. S. et al. ARTP/EMS-combined multiple mutagenesis efficiently improved production of raw starch-degrading enzymes in Penicillium oxalicum and characterization of the enzyme-hyperproducing mutant. Biotechnol. Biofuels 13, 187 (2020).
Zhao, S. et al. Combination of genetic engineering and random mutagenesis for improving production of raw-starch-degrading enzymes in Penicillium oxalicum. Microb. Cell Fact 21, 272 (2022).
Lin, H. J. et al. Production of raw cassava starch-degrading enzyme by Penicillium and its use in conversion of raw cassava flour to ethanol. J. Ind. Microbiol. Biotechnol 38, 733–742 (2011).
Xu, Q. S., Yan, Y. S. & Feng, J. X. Efficient hydrolysis of raw starch and ethanol fermentation: a novel raw starch-digesting glucoamylase from Penicillium oxalicum. Biotechnol. Biofuels 9, 216 (2016).
Hu, Y. et al. The interaction between the histone acetyltransferase complex Hat1-Hat2 and transcription factor AmyR provides a molecular brake to regulate amylase gene expression. Mol. Microbiol 119, 471–491 (2023).
Zhang, T. et al. Regulatory function of the novel transcription factor CxrC in Penicillium oxalicum. Mol. Microbiol 116, 1512–1532 (2021).
Zhao, S. et al. Simultaneous manipulation of transcriptional regulator CxrC and translational elongation factor eEF1A enhances the production of plant-biomass-degrading enzymes of Penicillium oxalicum. Bioresource Technol 351, 127058 (2022).
Pang, X. M. et al. G protein γ subunit modulates expression of plant-biomass-degrading enzyme genes and mycelial-development-related genes in Penicillium oxalicum. Appl. Microbiol. Biotechnol 105, 4675–4691 (2021).
Ma, B. et al. A mitogen-activated protein kinase PoxMK1 mediates regulation of the production of plant-biomass-degrading enzymes, vegetative growth, and pigment biosynthesis in Penicillium oxalicum. Appl. Microbiol. Biotechnol 105, 661–678 (2021).
Ma, B., Luo, X. M., Zhao, S. & Feng, J. X. Protein kinase PoxMKK1 regulates plant-polysaccharide-degrading enzyme biosynthesis, mycelial growth and conidiation in Penicillium oxalicum. J. Fungi (Basel) 9, 397 (2023).
Zhang, T. et al. Kinase POGSK-3β modulates fungal plant polysaccharide-degrading enzyme production and development. Appl. Microbiol. Biotechnol 107, 3605–3620 (2023).
He, Q. P. et al. Transcription factor NsdD regulates the expression of genes involved in plant biomass-degrading enzymes, conidiation, and pigment biosynthesis in Penicillium oxalicum. Appl. Environ. Microbiol 84, e01039–18 (2018).
Zhang, X. et al. Penicillium oxalicum putative methyltransferase Mtr23B has similarities and differences with LaeA in regulating conidium development and glycoside hydrolase gene expression. Fungal Genet. Biol 143, 103445 (2020).
Zhang, M. Y. et al. Identification of an essential regulator controlling the production of raw-starch-digesting glucoamylase in Penicillium oxalicum. Biotechnol. Biofuels 12, 7 (2019).
Ning, Y. N. et al. Regulation of fungal raw-starch-degrading enzyme production depends on transcription factor phosphorylation and recruitment of the Mediator complex. Commun. Biol 6, 1032 (2023).
Zhao, S. et al. Differential transcriptomic profiling of filamentous fungus during solid-state and submerged fermentation and identification of an essential regulatory gene PoxMBF1 that directly regulated cellulase and xylanase gene expression. Biotechnol. Biofuels 12, 103 (2019).
Wang, L. et al. Secretory overproduction of a raw starch-degrading glucoamylase in Penicillium oxalicum using strong promoter and signal peptide. Appl. Microbiol. Biotechnol 102, 9291–9301 (2018).
Li, C. X. et al. Three-dimensional genome map of the filamentous fungus Penicillium oxalicum. Microbiol. Spect 10, e0212121 (2022).
Yan, Y. S. et al. Transcriptomic profiling and genetic analyses reveal novel key regulators of cellulase and xylanase gene expression in Penicillium oxalicum. Biotechnol. Biofuels 10, 279 (2017).
Zhao, S. et al. Genetic modifications of critical regulators provide new insights into regulation modes of raw-starch-digesting enzyme expression in Penicillium. Biotechnol. Biofuels Bioprod. 2022 15, 62 (2017).
Cramer, P. Organization and regulation of gene transcription. Nature 573, 45–54 (2019).
Brückner, A., Polge, C., Lentze, N., Auerbach, D. & Schlattner, U. Yeast two-hybrid, a powerful tool for systems biology. Int. J. Mol. Sci 10, 2763–2788 (2009).
Pang, X. M. et al. G protein γ subunit modulates expression of plant-biomass-degrading enzyme genes and mycelial-development-related genes in Penicillium oxalicum. Appl. Microbiol. Biotechnol. 105, 4675–4691 (2021).
Adnan, M., Ma, X., Olsson, S., Wang, J. & Liu, G. Promoter regulation and genetic engineering strategies for enhanced cellulase expression in Trichoderma reesei. Microbiol. Res. 259, 127011 (2022).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Huber, W. et al. Orchestrating high-throughput genomic analysis with bioconductor. Nat. Methods 12, 115–121 (2015).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408 (2001).
Miller, G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem 31, 426–428 (1959).
Acknowledgements
J.X.F. acknowledges support for the research and publication of this work from the National Natural Science Foundation of China (grant numbers 32060141 and U21A20178). J.X.F. and S.Z. acknowledges support for the Key Research and Development Programme Project of Guangxi (Guike AB21076010). S.Z. acknowledges support for the Bagui Youth Talent Support Programme and the Training Program for 1000 Young and Middle-aged Key Teachers in Guangxi at 2019. Y.N.N. acknowledges support for the research from Innovation Project of Guangxi Graduate Education (grant number YCBZ2022046).
Author information
Authors and Affiliations
Contributions
S.Z. and J.X.F. conceived the study. Y.N.N. conducted mutation, tests of enzymatic production, RT-qPCR, and EMSA. X.L. carried out mutation, tests of enzymatic production, RT-qPCR, and Y1H assay. X.S. and W.T.L. performed measurement of enzymatic production and Y1H assay. D.T. performed RNA-seq analyses. S.Z., J.X.F., Y.N.N., and X.M.L. analyzed and interpreted the data. S.Z., J.X.F., and Y.N.N. wrote and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Syed Shams Yazdani and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Dr Silvia Belluti and Dr Ophelia Bu.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ning, YN., Liang, X., Shen, X. et al. A RsrC-RsrA-RsrB transcriptional circuit positively regulates polysaccharide-degrading enzyme biosynthesis and development in Penicillium oxalicum. Commun Biol 7, 848 (2024). https://doi.org/10.1038/s42003-024-06536-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06536-4
- Springer Nature Limited