Abstract
Oral squamous cell carcinoma (OSCC) is a serious public health problem in various Asian countries, including Sri Lanka, and a combination of cultural practices, lifestyle factors, and genetic predispositions influences the incidence of these cancers. The examination of the connection between exposure to heavy metals and the probability of developing oral potentially malignant disorders (OPMD) and OSCC has been limited in its scope, and the overall consequences of such exposure remain largely unknown. This study aims to clarify the link between serum levels of heavy metals and the risk of OSCC and OPMD. The concentrations of seven heavy metals—namely, arsenic (As), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), lead (Pb), and zinc (Zn)—were analyzed in serum samples from 60 cases and 15 controls in the Sri Lankan cohort. The Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) was used for the analysis. Subsequently, the data underwent statistical evaluation via the Kruskal–Wallis H test, using the Statistical Package for Social Sciences (SPSS) version 28 software, with a confidence interval set at 95%. A p-value less than 0.05 was considered statistically significant. The cohort consisted of 48 men and 27 women, with 15 patients each diagnosed with OSCC, OSF, OLK, and OLP, and 15 healthy controls. The study used the Kruskal–Wallis Test to compare metal concentrations across groups, finding significant differences for all metals except As and Pb. Significant associations were observed between age, past medical history, drug history, gender, smoking, alcohol consumption, and betel chewing. The Spearman Correlation test showed significant correlations between the concentrations of Cr, Co, Cu, As, and Zn and the presence of cancer/precancer conditions. The study’s findings suggest that heavy metal contamination may be linked to the development of OSCC and precancerous conditions. When comparing OSCC and OPMD cases with controls, the serum concentrations of As and Pb did not differ significantly. However, Cd, Cr, Co, Cu, and Zn exhibited significantly higher concentrations among cases compared to controls (p < 0.05). This study observed significant variations in the levels of these five heavy metals among cancerous (OSCC), premalignant (OPMD), and healthy tissues, suggesting a potential role in the progression of malignancies. These findings underscore the importance of environmental pollution in this specific context.
Similar content being viewed by others
Introduction
According to recent global estimates, lip and oral cavity malignancies, commonly known as “oral cancer,” rank as the 16th most prevalent type of cancer worldwide, with around 355,000 new cases reported annually1. Warnakulasuriya and Kerr, highlight that more than 90% of these cases involve squamous cell carcinomas, with over two-thirds occurring in economically disadvantaged nations, particularly in South Asia2. Oral squamous cell carcinoma (OSCC) frequently develops from various oral potentially malignant disorders (OPMDs), such as actinic cheilitis, discoid lupus erythematosus (DLE), erythroplakia, oral leukoplakia (OLK), oral lichen planus (OLP), or oral submucous fibrosis (OSF), which affect the oral mucosa and the lips. Histological examination of these lesions reveals varying degrees of structural and cellular abnormalities commonly referred to as “dysplasia” or “atypia”3. The primary factors influencing the emergence of OPMDs and their progression into OSCC include the consumption of areca nut, commercially prepared areca nut products like pan parag, mawa, babul beeda, and thul, as well as the combined practice of betel nut chewing with tobacco powder, such as Gutka. Additionally, tobacco is used in various forms, such as smoked tobacco (such as beedis, cigars, cigarettes, and shisha) and smokeless tobacco (snuff, snus, and chewable tobacco)4,5.
The association between heavy metals and cancer development, as well as the specific mechanisms underlying this relationship, remains incompletely understood. While knowledge exists regarding certain carcinogens such as chemical, physical, or viral agents, the role of heavy metals in tumor formation is not yet fully elucidated6,7,8,9,10,11. Metals such as Cd, As, Ni, and Cr have been classified by the International Agency for Research on Cancer (WHO–IARC) as carcinogenic to humans, placing them in Groups 1 and 212. Researchers in toxicogenomics are currently investigating various mechanisms related to heavy metal exposure, including angiogenesis, autophagy, DNA damage and repair, epigenetic alterations, genomic instability, inflammation, metabolic reprogramming, and oxidative stress7,11,13. Furthermore, even trace amounts of certain heavy metals can significantly influence the occurrence of double-strand breaks in DNA, leading to mutagenic alterations in the repair mechanism of body cells14.
Recent discussions have centered on the role of heavy metals in metastasis processes and their potential utility in developing new generations of medications and therapies against cancer and cancer metastasis15. Prolonged exposure to low levels of arsenic has been linked to various diseases, including pulmonary infections, hypertension and cardiovascular/neuromuscular diseases, diabetes mellitus, hepatic fibrosis, hepatic cirrhosis, hepatocellular carcinoma, melanosis, hyperkeratosis, and immunological disorders16. Furthermore, a study by Tsai et al. in 2017 found a significant correlation between elevated ambient nickel levels and an increased likelihood of developing OSCC and OPMDs9. Investigating iron and copper levels and their correlation with immune complexes in OPMDs and OSCC has been suggested as potential disease indicators17.
This study aimed to determine the correlation between heavy metals and the onset of OSCC. The hypothesis proposed in this investigation was that a positive association exists between heavy metal contamination and the development of OSCC and OPMD. The study examined the relationships among the levels of seven toxic metals in the blood—specifically arsenic As, Cd, Cr, Co, Cu, Pb, and Zn—and the risk of developing OSCC and OPMDs in a cohort of individuals from Sri Lanka.
Methods
Study population
The research was conducted at the Dental Teaching Hospital, University of Peradeniya, Sri Lanka, with the approval of the Ethical Review Committee, Faculty of Dental Sciences, University of Peradeniya (research project protocol number: ERC/FDS/UOP/E/2021/14). The study adhered to the Helsinki guidelines and regulations. Informed consent was obtained from all participants involved in this study, including both healthy controls (HC) and individuals with diseases. For healthy controls, participants were carefully selected through a comprehensive oral cavity examination conducted by an oral medicine specialist (AZH), who confirmed the absence of any pathological abnormalities. Exclusion criteria included individuals below the age of 18 and those with physical or mental incapacities that impeded their ability to participate in the required data-gathering procedures. The following data were collected from the study participants: age, gender, past medical history, drug history, and history of habits (Areca nut chewing, smoking, and alcohol consumption) (Table 1).
Serum heavy metals assay
A representative cohort of 60 cases was selected, comprising 15 individuals each with OSCC, OSF, OLP, and OLK, alongside 15 matched controls. The selection criteria included individuals diagnosed with OSCC or OPMD based on clinical or histopathological confirmation. Participants selected were ideally adult humans (age > 18), with no psychiatric issues or under medication. The samples were thawed at room temperature, mixed, and a 500 μL portion was transferred into acid-washed digestion tubes. Calibration standards for Cr, Co, Cu, Cd, As, Zn, and Pb were sourced from High Purity Standards in Charleston, South Carolina, USA, with concentrations of 1 mgL−1 for all except Zn, which was 5 mgL−1. These standards originated from the National Institute of Standards and Technology. A 500 μgL−1 concentration stock solution of Internal Standard was prepared from High Purity Standards in Charleston, South Carolina, USA.
Sample preparation and analytical processes employed concentrated nitric acid (Suprapur, Merck) with a volume-to-volume concentration of 65%, and ultrapure water with an 18 M Ohm cm−1 resistivity. Aliquots of 500 μL homogenate were transferred to acid-washed quartz vials, followed by the addition of 1.5 mL of nitric acid to each vial. The digestion process was conducted using the ONE TOUCH Technology, MARS6 digestion system, operating at 180 °C for 15 min. After cooling to room temperature, the solutions were diluted to a final volume of 10 mL using ultrapure water. Subsequently, the samples underwent analysis using the Thermo Scientific iCAP 7000 series ICP spectrometer technique. Multi-element working standards were prepared for each experimental trial by diluting standard stock solutions with a 2% (v/v) nitric acid solution. Daily calibration curves, consisting of eight points, were generated to ensure system accuracy across a concentration range. The concentrations were then calculated and recorded in micrograms per liter (μgL−1).
Statistical analysis
The research investigated variations in subject characteristics, such as age, gender, past medical history, and risk habits, by utilizing the chi-squared test to compare these factors among individuals diagnosed with OSCC, OPMD, and a control group. Due to the non-normal distribution of the dataset, we employed the Kruskal–Wallis H test, also known as the “one-way ANOVA on ranks,” a rank-based nonparametric test suitable for determining if there are statistically significant differences between two or more groups of an independent variable on a continuous or ordinal dependent variable. The analysis was conducted using Statistical Package for Social Sciences (SPSS) version 28 software, with a 95% confidence interval applied. A p-value of < 0.05 was considered statistically significant.
Ethics considerations
This study was conducted in accordance with the Helsinki Declaration of 1964 (as amended in October 2013 by the World Medical Association General Assembly). The study was carried out at the Dental Teaching Hospital, University of Peradeniya, Sri Lanka, with the approval of the Ethical Review Committee from the Faculty of Dental Sciences at the University of Peradeniya (research project protocol number: ERC/FDS/UOP/E/2021/14). All participants gave informed consent before participating in the study. Names, emails, or any other personal identifiers were not included in the data collected. Participation was informed and voluntary and the participant could withdraw from the study at any time.
Results
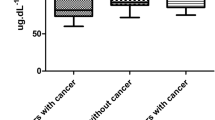
The study cohort comprised 75 individuals, with a slight male predominance (48 men and 27 women). Among them, 15 were diagnosed with OSCC, classified as follows: 6 with well-differentiated squamous cell carcinoma (SCC), 4 with moderately differentiated SCC, 3 with poorly differentiated SCC, 1 with early invasive SCC, and 1 with recurrent SCC. Additionally, there were 15 patients with OSF displaying varying degrees of dysplasia: 4 with mild to moderate epithelial dysplasia, 1 with moderate epithelial dysplasia with candida infection, and 10 with no epithelial dysplasia. Furthermore, 15 patients exhibited OLK, with 6 demonstrating keratosis alongside severe/moderate/mild epithelial dysplasia with candidal infection, 1 with mild/moderate epithelial dysplasia with candidal infection, 5 with keratosis with severe/moderate/mild epithelial dysplasia, 1 with keratosis without dysplasia and lichenoid reaction, and 1 with keratosis without dysplasia and keratosis with focal mild/mild epithelial dysplasia and 1 with verrucous hyperplasia with severe/moderate/mild epithelial dysplasia. The study also included 15 patients with OLP. Lastly, there were 15 healthy control subjects, meticulously matched for age, sex, and habits, who provided whole blood samples for the study (see Table 1 for specifics). Comprehensive descriptive statistics regarding the concentrations of heavy metals (measured in μg L−1) in sera samples from all subjects are outlined in Table 2.
Furthermore, the study identified specific associations within the cohort: age and past medical history showed a positive correlation (Pearson correlation = 0.520; p = 0.000), age and drug history exhibited a correlation (Pearson correlation = 0.420; p = 0.000), gender and smoking displayed a strong correlation (Pearson correlation = − 0.499; p = 0.000), gender and alcohol consumption revealed a correlation (Pearson correlation = − 0.685; p = 0.000), and age and betel chewing demonstrated a correlation (Pearson correlation = 0.406; p = 0.000).
This positive correlation between age and past medical history suggests that as age increases, the likelihood or extent of past medical history also increases. This is expected as older individuals tend to accumulate more medical history over time. In the same context, the positive correlation of age and drug history that older individuals are more likely to have a more extensive drug history.
The Kruskal–Wallis Test, a non-parametric statistical method, was employed to compare the medians of multiple groups that may not follow a normal distribution. Each metal’s associated p-value indicates whether there is a statistically significant difference in median concentrations between the cancer/precancer groups and the healthy control group. The findings show significant differences for all metals except As (p = 0.109) and Pb (p = 0.776) (refer to Table 3). Given that p < 0.001, strong evidence suggests a difference between at least one pair of groups; to determine which pair(s), pairwise comparisons were examined. Moreover, a Spearman Correlation test, which assesses the monotonic relationship between variables without assuming a linear connection, was performed (see Table 4). Each metal’s p-value indicates whether there is a statistically significant correlation between its concentration and the grouping (cancer/precancer or healthy control). The results indicate significant correlations for Cr, Co, Cu, As, and Zn. The average concentrations of heavy metals are visually depicted in Fig. 1.
Discussion
Our research unveiled a substantial increase in Cr, Co, Cu, Cd, and Zn levels in cancer and OPMD sera samples, which was statistically significant. Limited data exist on these heavy metals in OSCC and OPMD. Still, it is known that Cu and Zn serve as cofactors for superoxide dismutase (SODs) and participate in enzymes safeguarding cells against free radicals. The heightened Cu levels in our study’s cancerous tissues align with previous research18, linking excess Cu to direct DNA damage, potentially through reactive oxygen species. Moreover, these metallic ions play vital roles in angiogenesis, endothelial proliferation, and migration, which are crucial aspects of carcinogenesis18.
Zinc, crucial for cell growth, differentiation, apoptosis, and immune functions, exhibits accumulation in cancerous tissues. This ion influences mitogenic, and antioxidant activities19, and research suggests that a low zinc diet may enhance adenoma development, whereas a zinc-rich diet is linked to reduced cancer susceptibility20,21. In a relevant context, a recent study reported lower tissue and serum zinc levels in thyroid cancer patients compared to those in the control group, potentially indicating a connection to cancer development22. However, the zinc accumulation observed in cancerous and OPMD sera in our study lacks a precise explanation and necessitates further investigation into the etiologic correlation between OSCC and OPMD. Although our findings differ from those of Sohrabi et al. in 2017, where increased Zn levels were observed in colorectal cancer tissues, the exact cause for this variation remains unclear18. Potential factors contributing to this disparity may include the number of subjects, the analytical methodology employed, and actual distinctions among target populations. The irregular distribution of Zn in the cancerous condition may also play a role in this discrepancy. Consequently, additional research is warranted to gain a better understanding of the etiological correlation between zinc levels, OSCC, and OPMD18.
Our investigation also revealed heightened levels of Cr in cancerous tissues, adding to the ongoing discourse regarding its concentration in malignancies. The significance of Cr arises from its involvement in angiogenesis, production of reactive oxygen species, and subsequent damage to DNA through various signaling pathways, including p53, NF-κB, GADD45, Src kinase, and G proteins, which play pivotal roles in cell proliferation and differentiation23. Furthermore, Chiang et al. discovered a significant elevation in Cr concentration in the blood of OSCC patients compared to background levels, and it was positively correlated with the Cr concentration in the soil surrounding their residence (p-value < 0.023)24. Similarly, Sohrabi et al. demonstrated elevated Cr levels in cancerous tissues18. However, a discrepancy exists in the literature regarding the concentration of Cr in cancer tissues. While some studies report similar findings, others indicate lower concentrations of Cr in cancerous tissues compared to non-cancerous tissues25,26.
Our findings revealed relatively elevated levels of Co in samples from individuals with OLK and OSCC. In 1991, the International Agency for Research on Cancer conducted an assessment of the carcinogenic potential of Cobalt and its compounds27. The conclusion drawn was that there was insufficient evidence for carcinogenicity in humans, particularly concerning cancer of the lung. However, there was substantial evidence from experiments on animals. Many of the experimental studies considered employed exposure routes that were of questionable relevance for assessing the risk of human cancers. Examples included the development of local connective tissue cancers (sarcomas) after intramuscular injection. The overarching evaluation categorized cobalt and its compounds as possibly carcinogenic to humans. The assessment also observed that cobalt (II) compounds were found to induce damage to the DNA in vitro studies on both animal and human cells. Additionally, some evidence has suggested that these compounds could induce aneuploidy in vivo in Syrian hamster testes and bone marrow27,28,29.
However, unlike the other metals that show high concentrations in OSCC, Cd exhibits a higher concentration in OLK. Zhang et al. reported that male patients diagnosed with OSCC who engaged in betel quid chewing and smoking exhibited notably higher Cd levels in their dental calculus compared to healthy individuals without these habits30. This suggests a positive correlation between Cd levels and the risk of OSCC. Concurrently, a study found elevated Cd levels in the saliva of smokers compared to non-smokers; intriguingly, some OSCC patients, whether smokeless or non-drinkers, did not display signs of heavy metal poisoning in their oral mucosa31. The continuous stimulation of the oral mucosa by toxic heavy metals, particularly Cd, occurs during the chewing of betel quid and even when betel quid is not chewed. The porous and cellular structure of dental calculus allows heavy metals like Cd to leach from the calcified deposits. This continuous release of toxic trace heavy metals over extended periods, coupled with their stimulation of the gums, inner mouth lining, and tongue border, may contribute to the pathogenesis of OSCC30. Consequently, differences in the Cd content of dental calculus between individuals with and without OSCC might be evident32. Cadmium’s potential to cause toxicity and cancer arises from its ability to replace zinc in zinc finger DNA binding domains33. In vitro studies suggest that certain elements, including zinc, compete for transport mechanisms34. Cd has also been observed to displace zinc from various DNA repair enzymes, presenting a possible mechanism for Cd’s co-carcinogenicity35.
In general, the levels of trace elements found in OSCC and OPMDs may indicate both internal and external origins and could potentially influence cellular activities. Numerous studies have established connections between environmental pollutants such as Pb, Zn, Fe, and Mn and cancers36,37,38. It is crucial to consider the impact of cancer on the concentrations of these elements in the human body. Limited data exists regarding the concentrations of heavy metals in the sera of cancer and pre-cancer patients among those with OSCC. Larger-scale investigations are necessary to elucidate the specific role of each element in oral carcinogenesis. Our findings indicate that changes in these heavy metal element concentrations may contribute to the malignant transformation of normal oral mucosa.
Data availability
The authors confirm that the data supporting the findings of this study is available upon request from the corresponding author.
Abbreviations
- DLE:
-
Discoid lupus erythematosus
- ICP-OES:
-
Inductively Coupled Plasma-Optical Emission Spectrometry
- OLK:
-
Oral leukoplakia
- OLK:
-
Oral leukoplakia
- OLP:
-
Oral lichen planus
- OPMD:
-
Oral potentially malignant disorders
- OSCC:
-
Oral squamous cell carcinoma
- OSF:
-
Oral submucous fibrosis
- SCC:
-
Squamous cell carcinoma
- SOD:
-
Superoxide dismutase
References
Miranda-Filho, A. & Bray, F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 1(102), 104551 (2020).
Warnakulasuriya, S. & Kerr, A. R. Oral cancer screening: Past, present, and future. J. Dent. Res. 100(12), 1313–1320 (2021).
Rajendran, R. Shafer’s textbook of oral pathology (Elsevier India, 2009).
Amarasinghe, A. A., Usgodaarachchi, U. S., Johnson, N. W. & Warnakulasuriya, S. High prevalence of lifestyle factors attributable for oral cancer, and of oral potentially malignant disorders in rural Sri Lanka. Asian Pac. J. Cancer Prev.: APJCP 19(9), 2485 (2018).
Senevirathna, K., Jayasinghe, Y. A., Jayawickrama, S. M., Amarasinghe, H. & Jayasinghe, R. D. Oral cancer disease among the poor: A Sri Lankan context. Oral 3(3), 420–436 (2023).
Condoluci, A., Mazzara, C., Zoccoli, A., Pezzuto, A. & Tonini, G. Impact of smoking on lung cancer treatment effectiveness: A review. Future Oncol. 12(18), 2149–2161 (2016).
Koedrith, P., Kim, H., Weon, J. I. & Seo, Y. R. Toxicogenomic approaches for understanding molecular mechanisms of heavy metal mutagenicity and carcinogenicity. Int. J. Hyg. Environ. Health 216(5), 587–598 (2013).
Maret, W. The metals in the biological periodic system of the elements: Concepts and conjectures. Int. J. Mol. Sci.. 17(1), 66 (2016).
Tsai, K. Y., Su, C. C., Chiang, C. T., Tseng, Y. T. & Lian, I. B. Environmental heavy metal as a potential risk factor for the progression of oral potentially malignant disorders in central Taiwan. Cancer Epidemiol. 1(47), 118–124 (2017).
Viana, G. F., Garcia, K. S. & Menezes-Filho, J. A. Assessment of carcinogenic heavy metal levels in Brazilian cigarettes. Environ. Monit. Assess. 181, 255–265 (2011).
Wise, J. T., Wang, L., Zhang, Z. & Shi, X. The 9th conference on metal toxicity and carcinogenesis: The conference overview. Toxicol. Appl. Pharmacol. 15(331), 1–5 (2017).
IARC of WHO. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Last Updated: 2022–09–07. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 17 March 2023)
Wu, X. et al. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 23, 8244–8259 (2016).
Morales, M. E. et al. Heavy metal exposure influences double strand break DNA repair outcomes. PloS One 11(3), e0151367 (2016).
Fouani, L., Menezes, S. V., Paulson, M., Richardson, D. R. & Kovacevic, Z. Metals and metastasis: Exploiting the role of metals in cancer metastasis to develop novel anti-metastatic agents. Pharmacol. Res. 1(115), 275–287 (2017).
Ahmed, M. K., Habibullah-Al-Mamun, M., Parvin, E., Akter, M. S. & Khan, M. S. Arsenic induced toxicity and histopathological changes in gill and liver tissue of freshwater fish, tilapia (Oreochromis mossambicus). Exp. Toxicol. Pathol. 65(6), 903–909 (2013).
Tiwari, R. et al. Assessment of serum copper, iron and immune complexes in potentially malignant disorders and oral cancer. Braz. Oral Res. 10(30), e101 (2016).
Sohrabi, M. et al. Trace element and heavy metal levels in colorectal cancer: Comparison between cancerous and non-cancerous tissues. Biol. Trace Element Res. 183, 1–8 (2018).
Formigari, A., Gregianin, E. & Irato, P. The effect of zinc and the role of p53 in copper-induced cellular stress responses. J. Appl. Toxicol. 33(7), 527–536 (2013).
Bocca, B. et al. Determination of 30 elements in colorectal biopsies by sector field inductively coupled plasma mass spectrometry: Method development and preliminary baseline levels. Rapid Comm. Mass Spectrom. 21(11), 1776–1782 (2007).
Bocca, B., Mattei, D., Pino, A. & Alimonti, A. Italian network for human biomonitoring of metals: Preliminary results from two regions. Annali dell’Istituto superiore di sanità 46, 259–265 (2010).
Baltaci, A. K., Dundar, T. K., Aksoy, F. & Mogulkoc, R. Changes in the serum levels of trace elements before and after the operation in thyroid cancer patients. Biol. Trace Elem. Res. 175, 57–64 (2017).
Saghiri, M. A., Asatourian, A., Orangi, J., Sorenson, C. M. & Sheibani, N. Functional role of inorganic trace elements in angiogenesis—Part I: N, Fe, Se, P, Au, and Ca. Crit. Rev. Oncol. Hematol. 96(1), 129–142 (2015).
Chiang, C. T. et al. A critical exploration of blood and environmental chromium concentration among oral cancer patients in an oral cancer prevalent area of Taiwan. Environ. Geochem. Health 33, 469–476 (2011).
Alimonti, A. et al. A study on metals content in patients with colorectal polyps. J. Toxicol. Environ. Health Part A 71(5), 342–347 (2008).
Rinaldi, L. et al. Metals distribution in colorectal biopsies: New insight on the elemental fingerprint of tumour tissue. Dig. Liver Dis. 47(7), 602–607 (2015).
Cobalt and cobalt compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 52, 363–472 (1991). PMID: 1960848; PMCID: PMC7682369.
Lison, D., De Boeck, M., Verougstraete, V. & Kirsch-Volders, M. Update on the genotoxicity and carcinogenicity of cobalt compounds. Occup. Environ. Med. 58(10), 619–625 (2001).
Lison, D., Van Den Brûle, S. & Van Maele-Fabry, G. Cobalt and its compounds: Update on genotoxic and carcinogenic activities. Crit. Rev. Toxicol. 48(7), 522–539 (2018).
Zhang, B. et al. Evaluation of cadmium levels in dental calculus of male oral SCC patients with Betel-Quid Chewing in Hunan Province of China. Biol. Trace Elem. Research. 15(191), 348–353 (2019).
Talio, M. C., Luconi, M. O., Masi, A. N. & Fernández, L. P. Cadmium monitoring in saliva and urine as indicator of smoking addiction. Sci. Total Environ. 408(16), 3125–3132 (2010).
Tavakoli Pirzaman, A. et al. Toxic mechanisms of cadmium and exposure as a risk factor for oral and gastrointestinal carcinomas. Hum. Exp. Toxicol. 20(42), 09603271231210262 (2023).
Stohs, S. J., Bagchi, D., Hassoun, E. & Bagchi, M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 20(2), 12 (2001).
Drasch, G., Schöpfer, J. & Schrauzer, G. N. Selenium/cadmium ratios in human prostates: Indicators of prostate cancer risk of smokers and nonsmokers, and relevance to the cancer protective effects of selenium. Biol. Trace Elem. Res. 103, 103–107 (2005).
Schultze, B., Lind, P. M., Larsson, A. & Lind, L. Whole blood and serum concentrations of metals in a Swedish population-based sample. Scand. J. Clin. Lab. Investing. 74(2), 143–148 (2014).
Castiella, A. et al. Gender and plasma iron biomarkers, but not HFE gene mutations, increase the risk of colorectal cancer and polyps. Tumor Biol. 36(9), 6959–6963 (2015).
Qiao, L. & Feng, Y. Intakes of heme iron and zinc and colorectal cancer incidence: A meta-analysis of prospective studies. Cancer Causes Control 24, 1175–1183 (2013).
Spangler, J. G. & Reid, J. C. Environmental manganese and cancer mortality rates by county in North Carolina: An ecological study. Biol. Trace Elem. Res. 133, 128–135 (2010).
Acknowledgements
We thank the staff of the Diagnostic clinic and the Oral Maxillofacial Clinic, Department of Oral Medicine, Faculty of Dental Sciences, University of Peradeniya. and other Oral surgery and Oral Medicine colleagues who referred patients to this study.
Funding
This study was funded by the National Research Council of Sri Lanka (Grant number 22031), University Research Grants from Sri Jayewardenepura University of Sri Lanka (Grant number FMS/Q-12/2021), and a University Research Grant from the University of Peradeniya (D/F/CROC/URG/2022/29/D/CL/07).
Author information
Authors and Affiliations
Contributions
Study conception and design: KS, TANM, and RJ; Data analysis and interpretation: KS, PI, TANM; Writing original manuscript: KS; Writing reviewing and editing manuscript: KS, TANM, PI, NUJ, LJ, RW, KKK and CUG; Supervision: TANM, NUJ, CUG, LJ, RW, UP, BS, RJ and KKK; Publication funding acquisition: KKK; All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
Ruwan Duminda Jayasinghe is an editorial board member of Scientific Reports and a co-author of this article. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication. Other authors declare that they have no conflict of interest involved with their work in this study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Senevirathna, K., Mahakapuge, T.A.N., Ileperuma, P. et al. Correlation between serum heavy metals and the risk of oral squamous cell carcinoma and oral potentially malignant disorders. Sci Rep 14, 19029 (2024). https://doi.org/10.1038/s41598-024-70057-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70057-7
- Springer Nature Limited